Nuc-1031
CAS number: 840506-29-8
Fosgemcitabine Palabenamide is a small molecule drug with the largest clinical trial stage being Phase III (covering all indications) with six investigational indications.
Related images
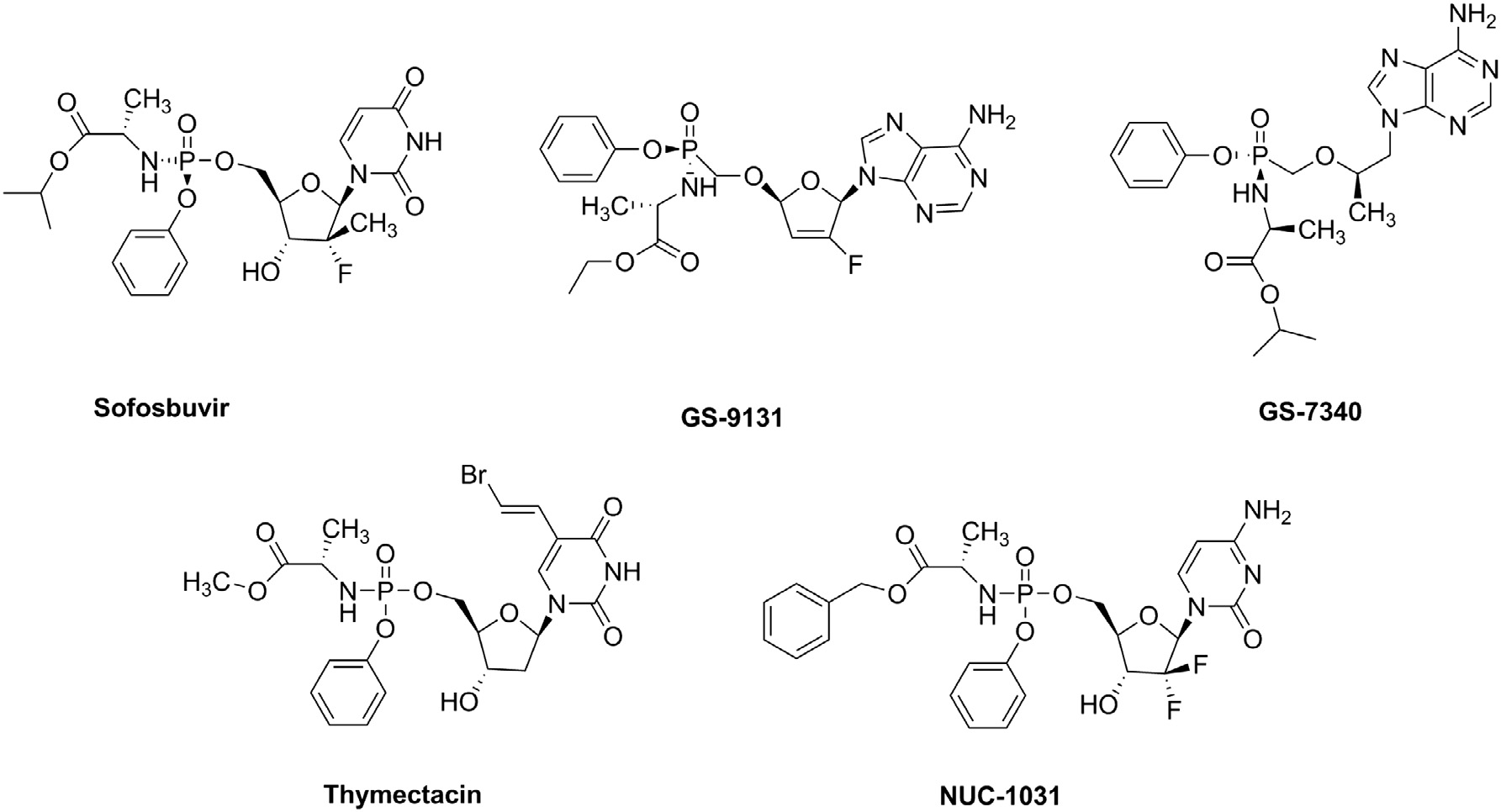
Known ProTides: SOFOSBUVIR, GS-9131, GS-7340, Thymectacin, NUC-1031.
Related Questions and Answers
A: The trial failed to show improved overall survival with NUC-1031 due to several factors. Firstly, there was a higher incidence of liver-related adverse events in the NUC-1031 arm, including elevated aminotransferase, bilirubin, and drug-induced liver injury (DILI). These toxicities likely led to reduced treatment exposure, with a median of 4 cycles in the NUC-1031 arm compared to 6 cycles in the control arm. Additionally, the objective response rate (ORR) was lower than expected, and the difference was not statistically significant. The study suggests that pre-existing liver impairment in aBTC patients may have exacerbated NUC-1031-induced liver injury.
A: NUC-1031 showed early promise in phase I trials with favorable safety and disease control in heavily pre-treated patients with solid tumors. However, in the phase III NuTide:121 trial, NUC-1031 combined with cisplatin did not improve overall survival compared to the standard gemcitabine/cisplatin regimen. It demonstrated a higher incidence of liver-related adverse events, leading to reduced treatment exposure and inferior overall survival. The study highlights the importance of thorough phase II evaluation before proceeding to phase III trials.
A: The combination of NUC-1031 and cisplatin significantly increases DNA damage and enhances cell cycle arrest compared to either agent alone. NUC-1031 causes prolonged S phase arrest and DNA damage response, while cisplatin primarily causes G2/M phase arrest. When combined, the drugs show additive effects on DNA damage markers such as p-ATM and γH2AX, and a synergistic effect on p-Chk1 phosphorylation. This suggests that the combination can lead to more effective cell cycle arrest and DNA damage, potentially enhancing the therapeutic efficacy in treating biliary tract and ovarian cancers.
A: NUC-1031 binds to the RRM1 subunit of ribonucleotide reductase, inhibiting its function and disrupting nucleotide pools. This binding can enhance DNA damage by reducing the availability of deoxynucleotides required for DNA replication. However, increased RRM1 expression in response to treatment can mitigate this effect. Clinically, RRM1 expression levels in tumor tissues have been suggested as a prognostic biomarker, with lower RRM1 expression correlating with better survival outcomes in some cancer types. However, the study found no conclusive correlation between RRM1 expression and survival across multiple cancers, likely due to the low number of biopsies and heavily pre-treated patient population.
A: NUC-1031 overcomes the limitations of gemcitabine by entering cancer cells independently of nucleoside transporters (hENT1 and hCNT1) and does not require activation by deoxycytidine kinase (dCK). Additionally, NUC-1031 is not subject to breakdown by cytidine deaminase (CDA), allowing it to produce higher concentrations of the active metabolite dFdCTP inside tumor cells. This results in prolonged DNA incorporation and sustained DNA damage, enhancing its anticancer activity.
A: NUC-1031 is metabolized to dFdCTP, which is incorporated into DNA, causing cell cycle arrest in the S phase and eliciting a DNA damage response. When combined with cisplatin, the DNA damage is increased and occurs earlier compared to monotherapy. The combination also affects nucleotide pools by binding to the RRM1 subunit of ribonucleotide reductase, potentially enhancing the DNA damage response. This mechanism suggests that the combination of NUC-1031 and cisplatin can lead to synergistic effects, enhancing the efficacy of treatment in biliary tract and ovarian cancer.