Klason lignin
CAS number: 8068-04-0
Klason lignin is a method for isolating lignin from biomass by treating it with strong sulfuric acid to hydrolyze and remove carbohydrates, leaving behind the acid-insoluble lignin. This method is commonly used to determine the total lignin content of plants, particularly in wood and pulp. It's also referred to as acid-insoluble lignin.
Related images
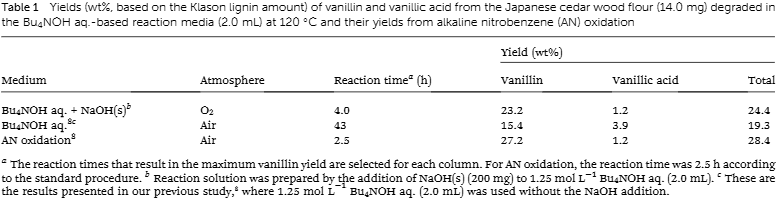
Yields (wt%, based on the Klason lignin amount) of vanillin and vanillic acid from the Japanese cedar wood flour (14.0 mg) degraded in the Bu4NOH aq.-based reaction media (2.0 mL) at 120 ℃ and their yields from alkaline nitrobenzene (AN) oxidation
Related Questions and Answers
A: The reaction mechanism of Klason lignin in a lithium battery system involves several steps. Initially, lithium ions interact with carbonyl groups (C=O) in lignin, resulting in a voltage plateau from 3.3 to 1.5V. Further discharge leads to the reduction of hydroxyl groups (–OH), causing a voltage drop from 1.5 to 1V. Finally, interactions with ether groups (C–O–C) occur below 1V. These reactions are irreversible, contributing to the overall specific capacity of the battery.
A: The electrochemical properties of Klason lignin were characterized using several techniques. The conductivity of Klason lignin was found to be 10–9S cm–1, indicating it is an insulator. The specific capacity of the Li/KL cell reached 380mAh g–1 at a current density of 25mA g–1. The galvanostatic discharge curve showed two voltage plateaus, corresponding to different electrochemical reactions involving carbonyl and hydroxyl groups. Cyclic voltammetry confirmed these reactions, with peaks at 2V, 1.4V, and 0.7V corresponding to interactions with different functional groups in lignin.
A: The oxygen content in Klason lignin extracted from sunflower husks is higher (40wt.%) compared to hydrolytic lignin (23wt.%). This higher oxygen content makes Klason lignin more promising as a cathode-active material because it can provide more sites for electrochemical reactions with lithium ions, leading to higher specific capacity.
A: Klason lignin extracted from sunflower husks can be used as a cathode-active material in primary lithium batteries. The study demonstrated that it has a maximum specific capacity of 380mAh g–1 at a current density of 25mA g–1, showing good prospects for use in low-rate, long-term power sources.
A: Torrefaction at 583 K for 50 minutes results in wood samples that are significantly enriched in lignin, with decomposition behaviors that closely match those of Klason lignin. The thermogravimetric curves of residual lignin in torrefied wood and Klason lignin are almost identical above 583 K, confirming that Klason lignin can be used as a good model compound for native lignin in wood. This finding is important for developing accurate kinetic models for the pyrolysis of torrefied wood.
A: The kinetic parameters for the decomposition of Klason lignin isolated from beech wood are as follows:
First Step: Activation energy (E1) = 60.9 kJ/mol, Pre-exponential factor (A1) = 5.8 × 10^4 s^-1, Reaction order (n1) = 3.0, Fraction of volatile products (ν1) = 0.16
Second Step: Activation energy (E2) = 147.4 kJ/mol, Pre-exponential factor (A2) = 8.2 × 10^10 s^-1, Reaction order (n2) = 3.4, Fraction of volatile products (ν2) = 0.26
Third Step: Activation energy (E3) = 112.9 kJ/mol, Pre-exponential factor (A3) = 3.2 × 10^5 s^-1, Reaction order (n3) = 2.0, Fraction of volatile products (ν3) = 0.074
These parameters accurately describe the thermogravimetric behavior of beech wood Klason lignin over a wide temperature range, with deviations between measured and simulated curves being very low (devTG = 0.9%, devDTG = 2.8%).
First Step: Activation energy (E1) = 60.9 kJ/mol, Pre-exponential factor (A1) = 5.8 × 10^4 s^-1, Reaction order (n1) = 3.0, Fraction of volatile products (ν1) = 0.16
Second Step: Activation energy (E2) = 147.4 kJ/mol, Pre-exponential factor (A2) = 8.2 × 10^10 s^-1, Reaction order (n2) = 3.4, Fraction of volatile products (ν2) = 0.26
Third Step: Activation energy (E3) = 112.9 kJ/mol, Pre-exponential factor (A3) = 3.2 × 10^5 s^-1, Reaction order (n3) = 2.0, Fraction of volatile products (ν3) = 0.074
These parameters accurately describe the thermogravimetric behavior of beech wood Klason lignin over a wide temperature range, with deviations between measured and simulated curves being very low (devTG = 0.9%, devDTG = 2.8%).
A: Klason lignin from agricultural residues (olive pomace and wheat straw) shows wider zones of high devolatilization rates at lower temperatures compared to wood lignin. The peak rates for agricultural residues are attained at around 600–620 K, while hardwood lignin peaks at around 645 K and softwood lignin at around 680 K. The agricultural residue lignins also exhibit a shoulder zone at higher temperatures, indicating the decomposition of different lignin fragments. Despite these differences, a three-step kinetic model can describe the decomposition behavior of both wood and agricultural residue lignins, with similar activation energies and reaction orders.
A: Klason lignin serves as a reliable model compound for studying the pyrolysis behavior of lignin in lignocellulosic biomass. Its decomposition characteristics closely mimic those of residual lignin in torrefied woods, making it useful for formulating accurate kinetic models for biomass pyrolysis. The study found that a three-step kinetic model can accurately describe the decomposition of Klason lignin over a wide temperature range (300–973 K), with activation energies and reaction orders that are consistent across different origins (wood and agricultural residues). This model can be extended to other feedstocks and isolation methods, improving the understanding and prediction of lignin decomposition during pyrolysis.