9,10-Dihydrophenanthrene
CAS number: 776-35-2
9,10-Dihydrophenanthrene is a chemical compound, specifically a derivative of phenanthrene. It's a hydrogenated form of phenanthrene, meaning two hydrogen atoms have been added to the phenanthrene structure, breaking one of the double bonds in the aromatic ring system. This hydrogenation occurs at the 9 and 10 positions of the phenanthrene molecule.
Related images
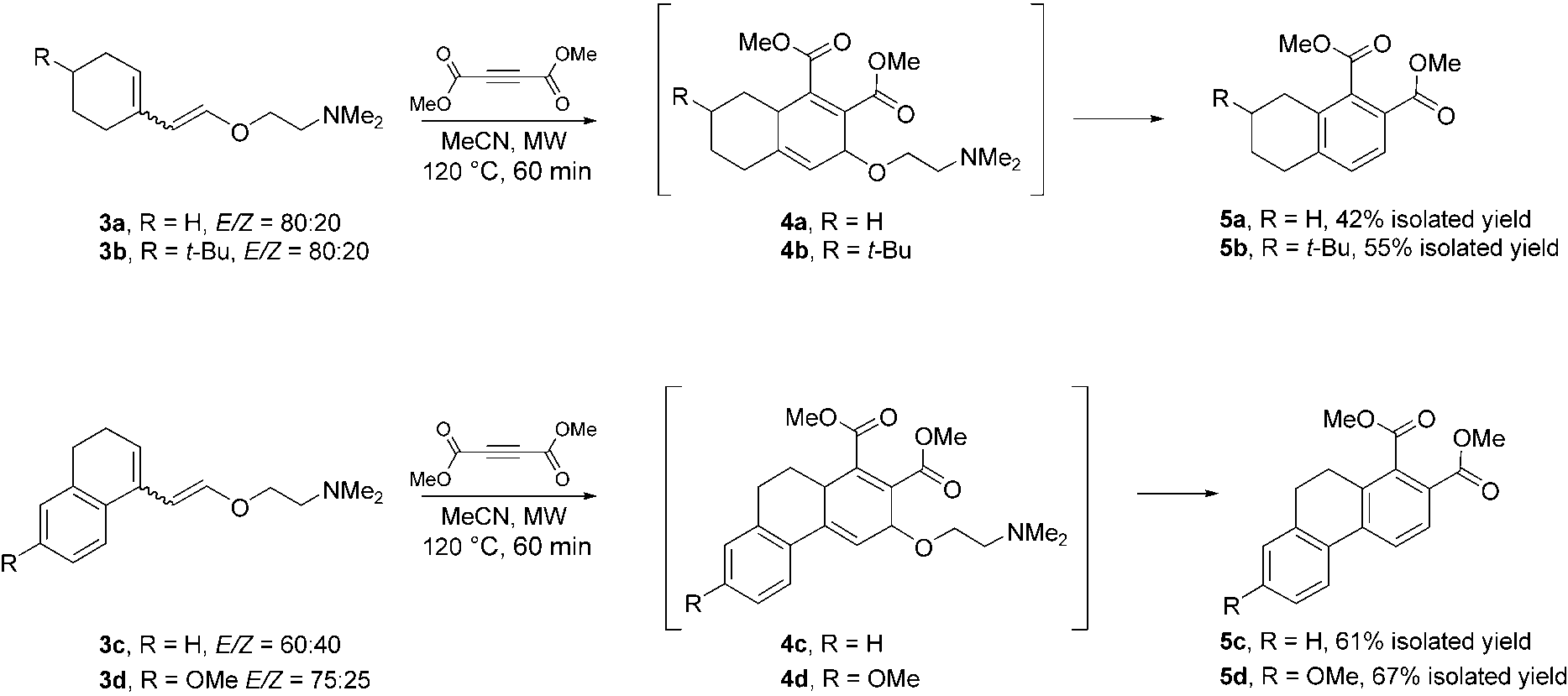
microwave-mediated synthesis of tetrahydronaphthalene and dihydrophenanthrene derivatives via Diels–Alder intermediates.
Related Questions and Answers
A: The study isolated a new compound, 9,10-dihydro-5-methoxy-8-methyl-2,7-phenanthrenediol (1), and two new optically active compounds, (2S,4'R,8'R)-3,4-δ-dehydrotocopherol (2) and (2R,4'R,8'R)-3,4-δ-dehydrotocopherol (3). The structures of these compounds were determined using spectroscopic data analysis. Compound 1 is a 9,10-dihydrophenanthrene derivative with methoxy and methyl groups at specific positions. Compounds 2 and 3 are stereoisomers of 3,4-δ-dehydrotocopherol, with absolute configurations at C-2 determined by Circular Dichroism (CD) experiments.
A: The hydrocracking of 9,10-DHP is initiated through two primary routes: bimolecular hydrogen transfer and monomolecular protolytic attack on the central rings. The key finding is that the relative influence of these two hydrocracking pathways is predominantly governed by the pore topological structure of the zeolites. Specifically:
H-ZSM-5 (10MR pores): Predominantly favors the monomolecular protolytic attack pathway, leading to extensive hydrogenation and cracking of 9,10-DHP into small alkanes (propane, butanes, pentanes, etc.). This is due to its small pore structure, which facilitates deep cracking.
H-USY (12MR pores): Favors the bimolecular hydrogen transfer pathway, resulting in the formation of phenanthrenes, tetrahydropenanthrene (THP), and other aromatic compounds. This is attributed to its larger pore size, which allows for bimolecular reactions.
H-Beta (12MR pores with a smaller sinusoidal channel): Exhibits a hybrid performance characteristic of both small-pore and large-pore zeolites. It shows a higher proportion of small molecular alkanes and benzene compared to H-USY, due to its unique pore structure that facilitates both protolytic cracking and dealkylation reactions.
H-ZSM-5 (10MR pores): Predominantly favors the monomolecular protolytic attack pathway, leading to extensive hydrogenation and cracking of 9,10-DHP into small alkanes (propane, butanes, pentanes, etc.). This is due to its small pore structure, which facilitates deep cracking.
H-USY (12MR pores): Favors the bimolecular hydrogen transfer pathway, resulting in the formation of phenanthrenes, tetrahydropenanthrene (THP), and other aromatic compounds. This is attributed to its larger pore size, which allows for bimolecular reactions.
H-Beta (12MR pores with a smaller sinusoidal channel): Exhibits a hybrid performance characteristic of both small-pore and large-pore zeolites. It shows a higher proportion of small molecular alkanes and benzene compared to H-USY, due to its unique pore structure that facilitates both protolytic cracking and dealkylation reactions.
A: The SAR studies revealed that the presence of bulkier groups at the R1 position, such as cyclohexyl and 4-Br phenyl, improved inhibitory activity. The conversion of the pyridine group to quinoline at the R3 position was favorable, while pyrimidine, pyrazole, and purine derivatives were unfavorable. The incorporation of 5-phenyl at the pyridyl group was crucial for high inhibitory activity.
A: The 9,10-dihydrophenanthrene derivatives were synthesized using a rhodium (III)-catalyzed C-H activation and relay Diels-Alder reaction. The compounds were tested for their inhibitory activity against SARS-CoV-2 3CLpro using a FRET assay. The most potent compounds, C1 and C2, were further evaluated for their inhibitory mechanisms and metabolic stability.
A: The 9,10-dihydrophenanthrene derivatives act as non-covalent inhibitors of the SARS-CoV-2 3CLpro, effectively inhibiting viral replication. Compounds C1 and C2 showed the most potent inhibitory activity, with IC50 values of 1.55±0.21 mM and 1.81±0.17 mM, respectively. These compounds have potential for further development as orally administered antiviral agents.