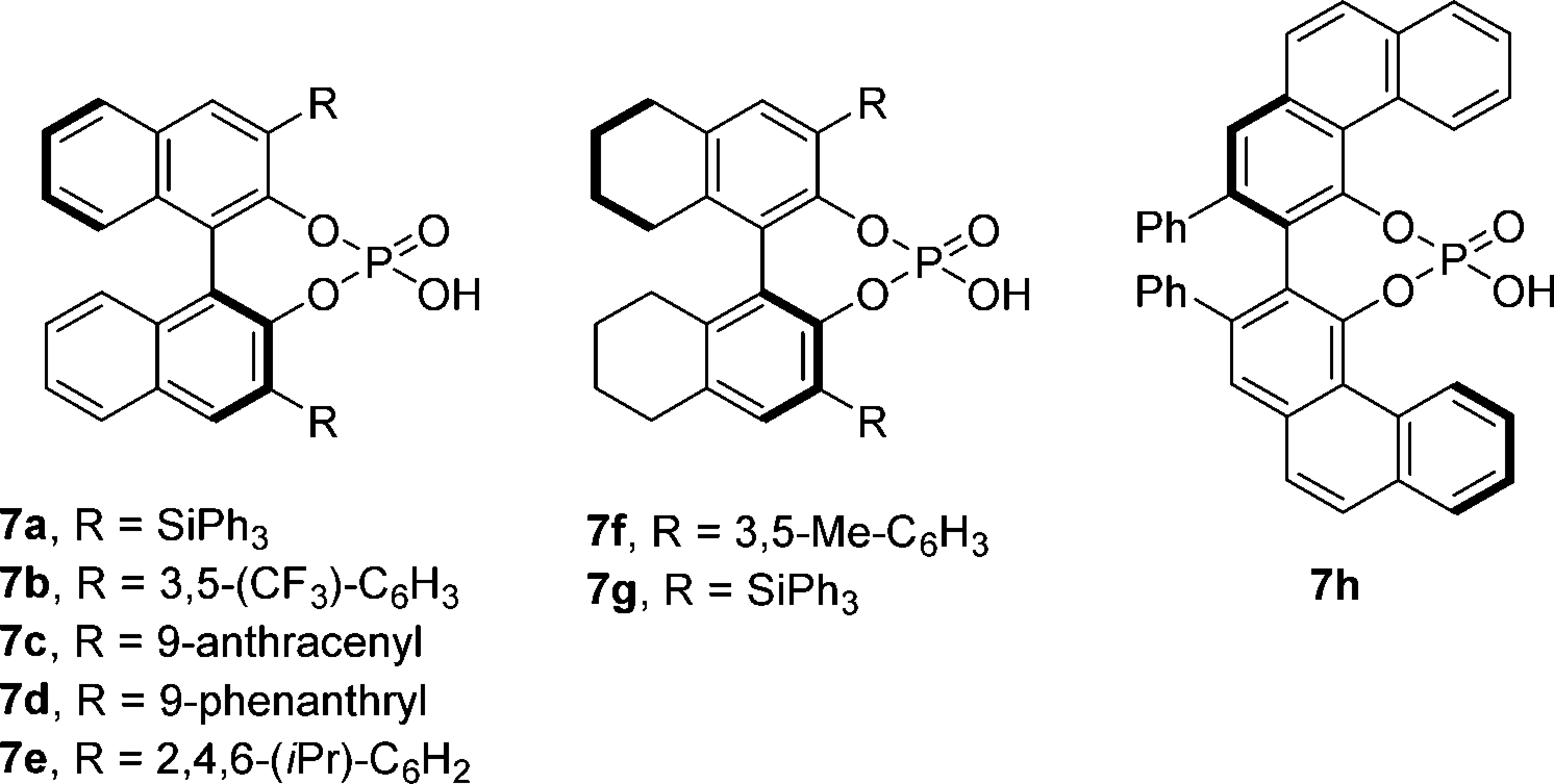
Phosphoric acid
CAS number: 7664-38-2
Phosphoric acid (H3PO4) is a colorless syrupy liquid. It is also known as orthophosphoric acid and its available analytical grade is 85%. Phosphoric acid is made from the mineral phosphorus, which is found naturally in many foods.
Related images
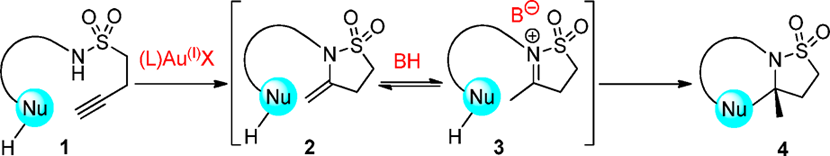
Concept of Dual Catalytic Gold and Organic Phosphoric Acid Enantioselective Cyclization Cascade
![N,N-diallyl-p-toluenesulfonamide (5) was cycloisomerized in [D8]toluene (0.17 m) at 50 °C in the presence of B (5 mol%) and 2b (1 equiv). The relative contents of B, G and decomposition products were estimated by 31P NMR spectroscopy with H3PO4 (85%) as external standard and 1,2;5,6-dibenzanthracene (5 mol%) as internal standard.](http://www.wlxkc.cn/picture/1560075_38.png)
N,N-diallyl-p-toluenesulfonamide (5) was cycloisomerized in [D8]toluene (0.17 m) at 50 °C in the presence of B (5 mol%) and 2b (1 equiv). The relative contents of B, G and decomposition products were estimated by 31P NMR spectroscopy with H3PO4 (85%) as external standard and 1,2;5,6-dibenzanthracene (5 mol%) as internal standard.
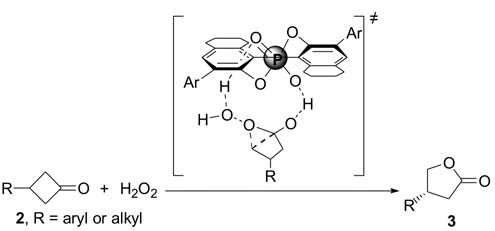
BINOL-derived chiral phosphoric acid-catalyzed enantioselective B-V reaction of 3-substituted cyclobutanones
Related Questions and Answers
A: After electropolishing with phosphoric acid for 5 minutes, the surface morphology of the spherical particles becomes less and smaller, but the surface is not yet flat and smooth. Electropolishing with perchloric acid for 5 minutes results in a flatter and smoother surface. As the electropolishing time increases to 7 and 10 minutes, the surface roughness decreases further, with the surface becoming relatively smooth after 10 minutes of electropolishing with perchloric acid. The surface roughness decreases from 11.69 μm (untreated) to 1.76 μm (10 minutes with perchloric acid).