Methanesulfonic acid
CAS number: 75-75-2
Methanesulfonic acid is an alkanesulfonic acid in which the alkyl group directly linked to the sulfo functionality is methyl. Methanesulfonic acid with high acidity is not only the catalyst in the process of chitin acylation, but also is a good solvent for partially acylated chitin.
Related images
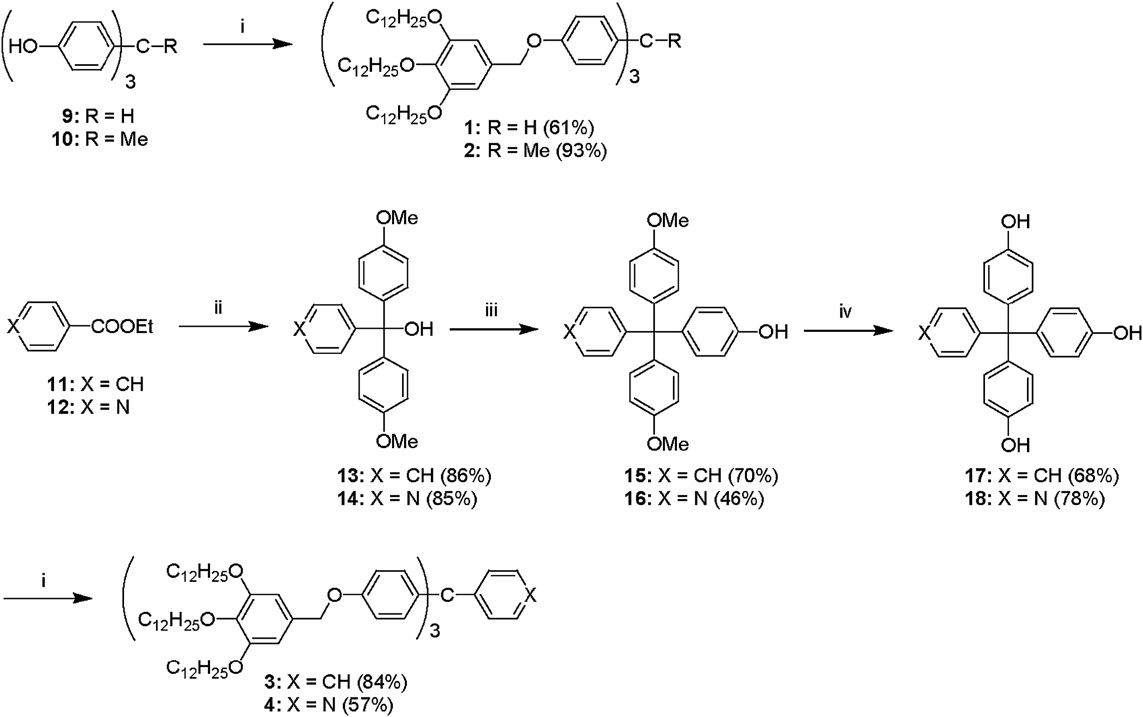
Synthesis of liquid crystals 1–4: (i) 3,4,5-tridodecyloxybenzyl chloride, K2CO3, DMF; (ii) 4-bromoanisole, Mg, THF; (iii) phenol, methanesulfonic acid; (iv) BBr3, CH2Cl2.
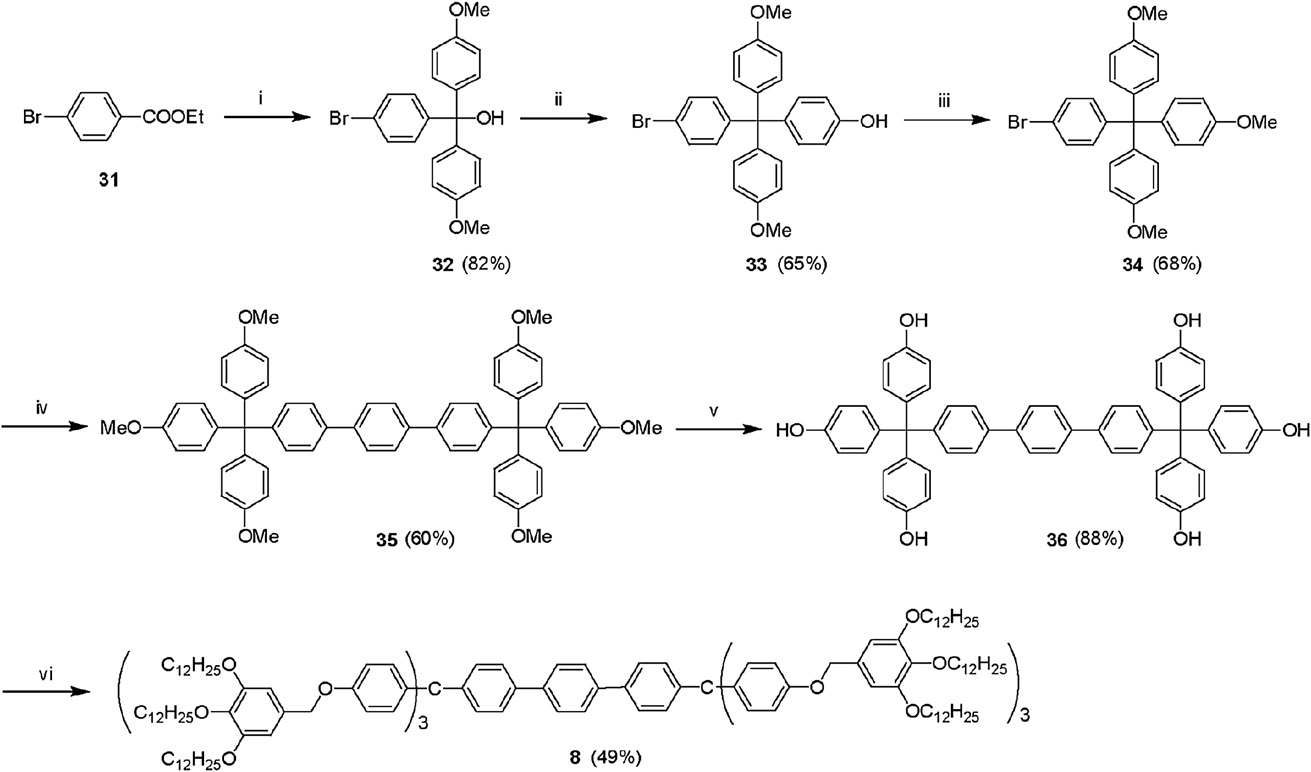
Synthesis of the liquid crystal 8: (i) 4-bromoanisole, Mg, THF; (ii) phenol, methanesulfonic acid; (iii) CH3I, K2CO3, DMF; (iv) 1,4-phenyldiboronic acid, Pd(PPh3)4, Na2CO3, THF, H2O; (v) BBr3, CH2Cl2; (vi) 3,4,5-tridodecyloxybenzyl chloride, K2CO3, DMF.
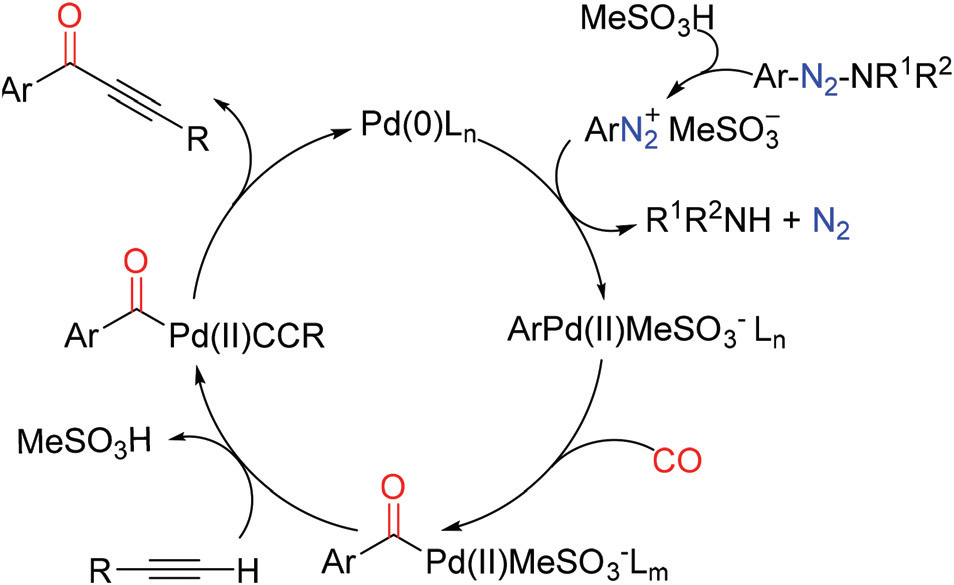
First, the aryl triazene is converted to the corresponding aryl diazonium salt with the help of methanesulfonic acid. Then, the generated aryl diazonium salt undergoes oxidative addition with Pd(0) to obtain an aryl palladium complex, which, after coordination and insertion of a CO molecule, gives an acyl palladium intermediate. The terminal alkyne is then generated, and a new Pd-C bond is formed with the help of MeSO3−, which gives the desired alkynone after reductive elimination and provides Pd(0) for the next catalytic cycle. In this process, methanesulfonic acid may play three roles: (1) the aryl triazene generates an aryl diazonium salt; (2) MeSO3 deprotonates the terminal alkyne and re-forms methanesulfonic acid; (3) the regenerated methanesulfonic acid reacts with the previously released free amine, avoiding the nucleophilic attack of the amine on the acyl palladium intermediate and generating the non-target amide.
Related Questions and Answers
No related questions yet