Acetonitrile
CAS number: 75-05-8
Acetonitrile is a toxic, colorless liquid with an ether-like odor and a sweet, burnt taste. It is extremely dangerous and can cause severe health effects.
Related images
![Cyclic voltammetry performed at a gold-disk electrode (d = n1 mm) at 22 °C in acetonitrile (S) containing Bu4NBF4 (0.3 M) as the supporting electrolyte: (a) Reduction of [FeIICl2(S)4] (4S; 4 mM) at a scan rate of 0.5 V s−1; (b) [FeIICl2(S)4] (4S; 4 mM) in the presence of NaBH4 (4 equiv) at a scan rate of 5 V s−1; (c) reduction of isolated [(η1-H3BH)FeIICl(S)4] (5; 4 mM) at a scan rate of 0.5 V s−1.](http://www.wlxkc.cn/picture/947257_11.png)
Cyclic voltammetry performed at a gold-disk electrode (d = n1 mm) at 22 °C in acetonitrile (S) containing Bu4NBF4 (0.3 M) as the supporting electrolyte: (a) Reduction of [FeIICl2(S)4] (4S; 4 mM) at a scan rate of 0.5 V s−1; (b) [FeIICl2(S)4] (4S; 4 mM) in the presence of NaBH4 (4 equiv) at a scan rate of 5 V s−1; (c) reduction of isolated [(η1-H3BH)FeIICl(S)4] (5; 4 mM) at a scan rate of 0.5 V s−1.
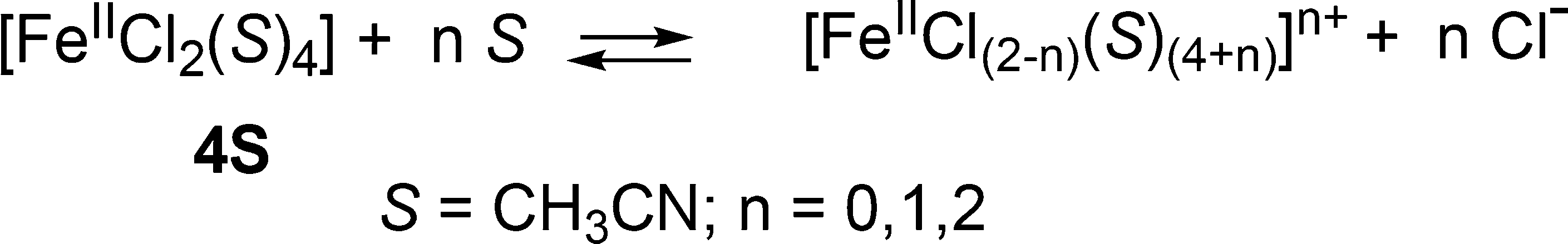
Postulated Iron(II) Complexes Formed in Acetonitrile
![Synthesis of [(η1-H3BH)FeCl(NCCH3)4] (5) by Reaction of NaBH4 with {Fe(μ-Cl)2(NCCH3)2}n in Acetonitrile](http://www.wlxkc.cn/picture/947257_13.png)
Synthesis of [(η1-H3BH)FeCl(NCCH3)4] (5) by Reaction of NaBH4 with {Fe(μ-Cl)2(NCCH3)2}n in Acetonitrile
 in Acetonitrile](http://www.wlxkc.cn/picture/947257_15.png)
Distribution of (a) Mono- and (b) Bis-Borohydrido FeII Species Obtained by Reaction of Excess NaBH4 with [FeCl2(S)4](4S) in Acetonitrile
2 in MeCN was 1.17 × 10−5 M. The lifetime of the photocatalyst in the excited state τ = 890 ns in MeCN.](http://www.wlxkc.cn/picture/1475256_24.png)
Stern–Volmer quenching experiments of Ru(bpy)3 for 4a, 4b, and 5a. The concentration of [Ru(bpy)3](PF6)2 in MeCN was 1.17 × 10−5 M. The lifetime of the photocatalyst in the excited state τ = 890 ns in MeCN.
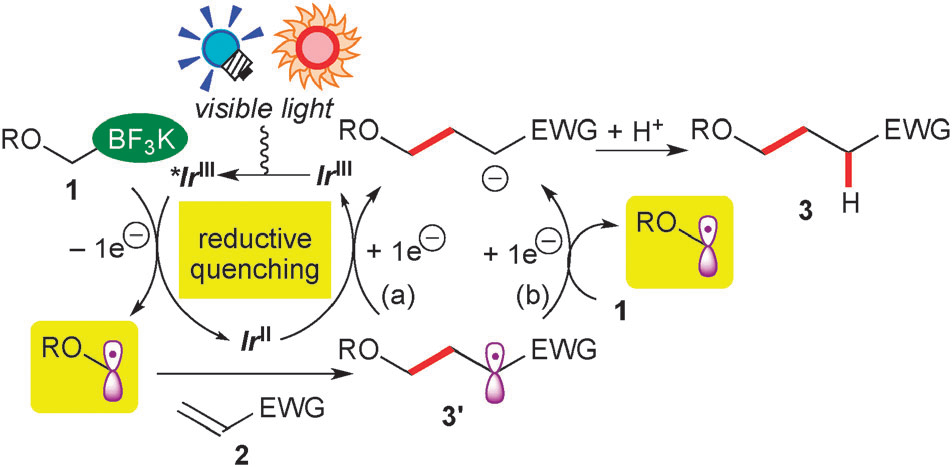
A plausible reaction mechanism.

a) UV–vis absorption spectroscopy of DHA 1 and VHF 2 in MeCN. Irradiation of DHA 1 (1.0 × 10−5 M) with UV light (365 nm) induces ring-opening reaction to VHF 2. The initial DHA 1 and final VHF 2 absorption spectra are indicated by thick lines. VHF 2 undergoes a thermally-induced ring-closure reaction back to DHA 1. b) Raman spectra of solid-state powders composed of DHA 1 only, a mixture of DHA 1 and VHF 2, and VHF 2 only. c) Upon UV exposure, the color of the powders changed from transparent to deep red. Initially bright blue fluorescence was also observed which rapidly disappeared during photoconversion. The scale bar is 20 μm.
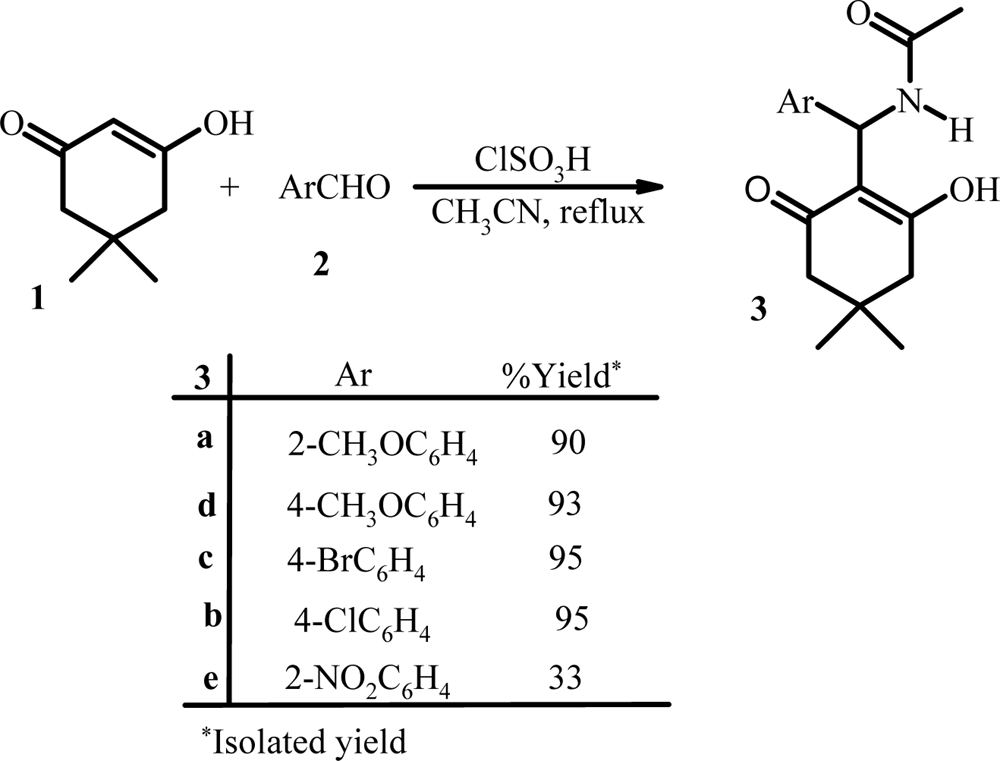
Three-component reaction of dimedon, aromatic aldehydes, and acetonitrile.
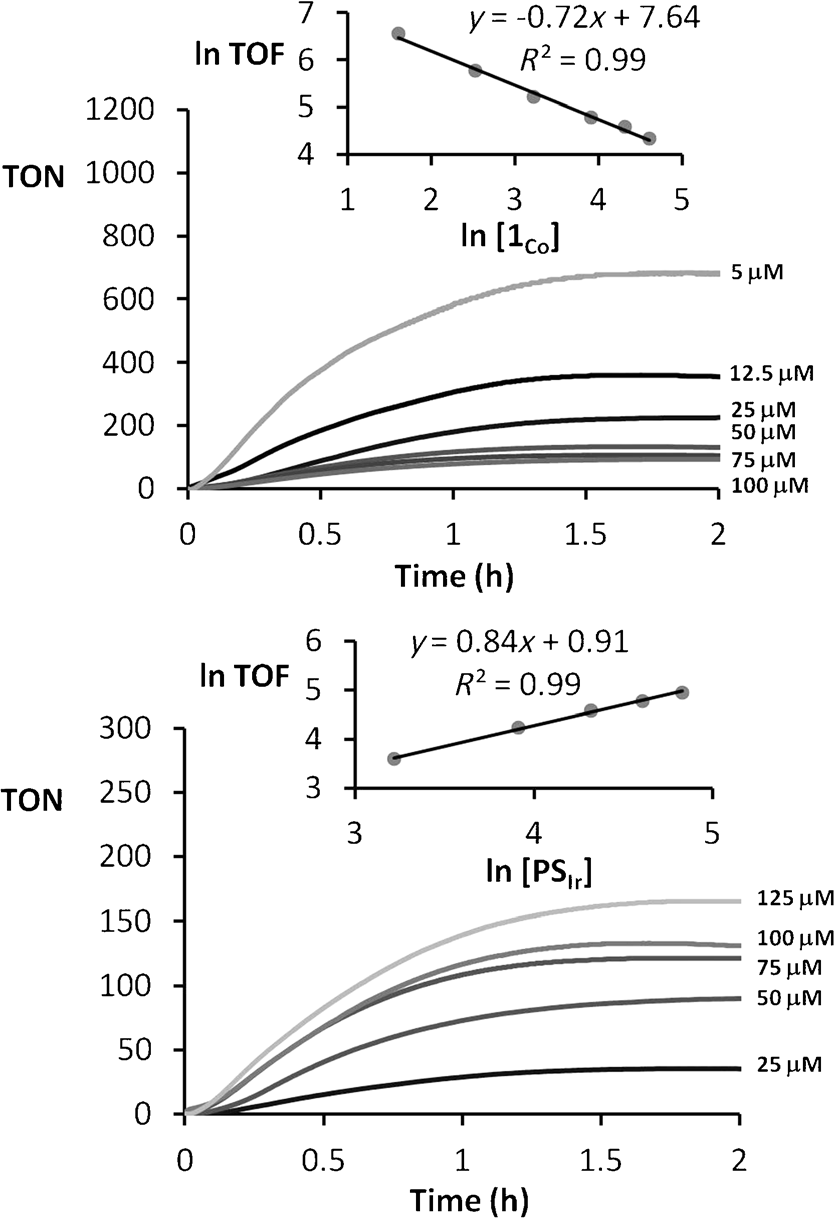
Plots of on-line pressure monitored versus time for reactions using CH3CN/H2O/Et3N (2:8:0.2) as solvent mixture under irradiation at l=447 nm.
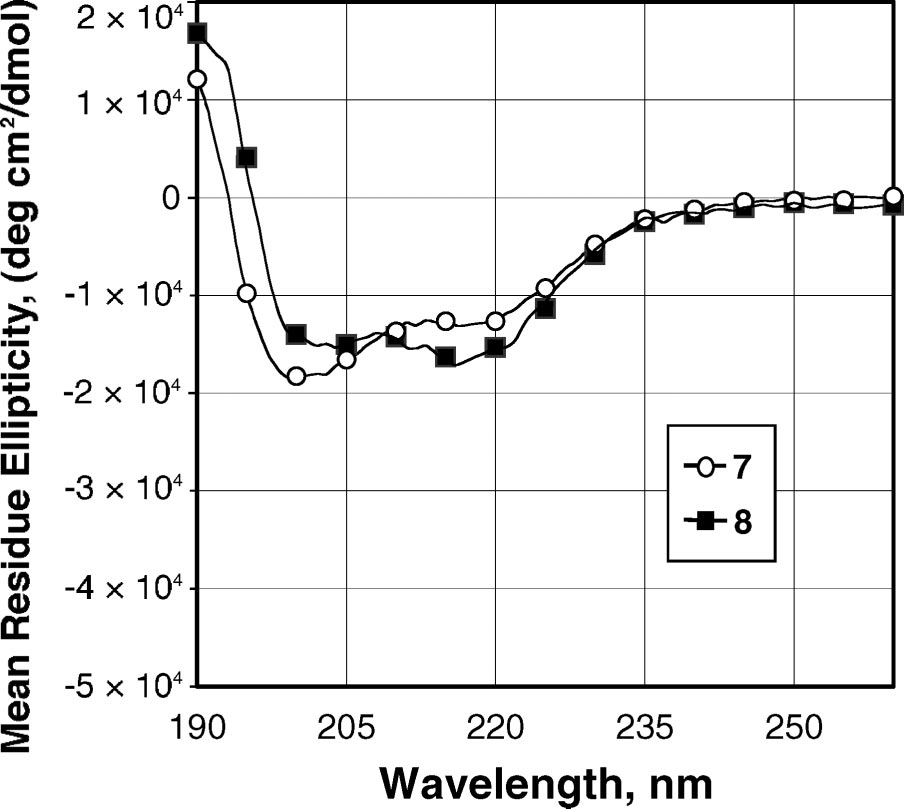
CD spectra of peptoids 7 and 8 at 60 µM in acetonitrile.
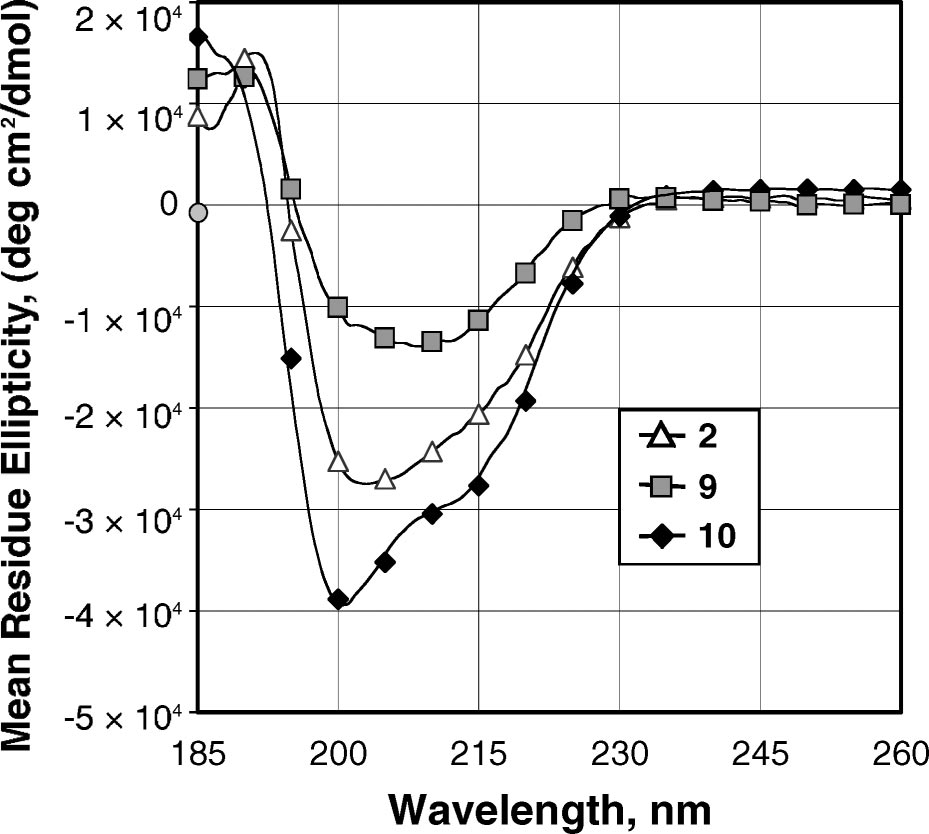
CD spectra of peptoids 2, 9, and 10 at 60 µM in acetonitrile.
Related Questions and Answers
No related questions yet