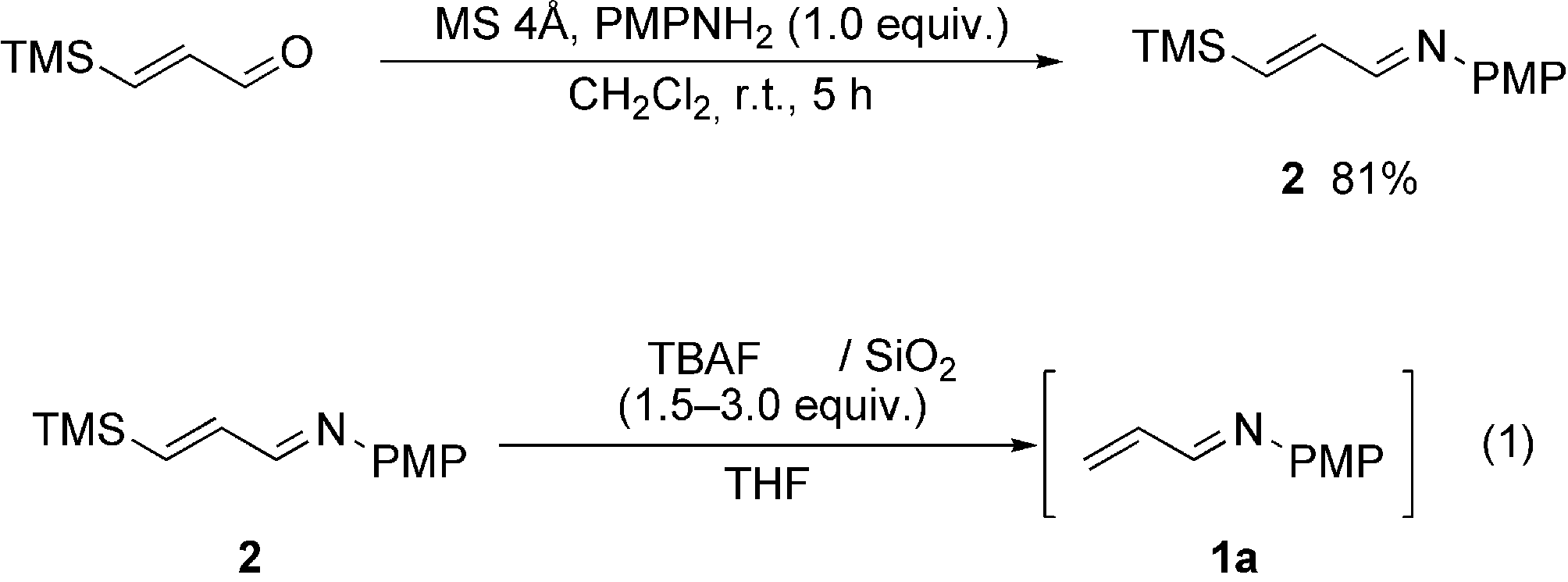
Acrolein
CAS number: 107-02-8
Acrolein is a colorless or yellow liquid with a disagreeable odor. It dissolves in water very easily and quickly changes to a vapor when heated. It also burns easily. Small amounts of acrolein can be formed and can enter the air when trees, tobacco, other plants, gasoline, and oil are burned. Acrolein is used as a pesticide to control algae, weeds, bacteria, and mollusks. It is also used to make other chemicals.
Related images
Related Questions and Answers
A: Accurate analysis of acrolein is challenging because of its high reactivity and instability, leading to its propensity to polymerize and form adducts with various cellular components such as proteins, DNA, and antioxidants, meaning it often exists in a bound form rather than as free acrolein within complex food matrices. To overcome these challenges, derivatization techniques are integrated with analytical methods like liquid chromatography-tandem mass spectrometry (LC-MS/MS). Common derivatization reagents include 2,4-dinitrophenylhydrazine (2,4-DNPH) and pentafluorophenylhydrazine (PFPH), which increase the molecular weight and reduce the volatility of acrolein for reliable analysis.
A: While heat-induced reactions are major contributors to acrolein formation, this study reveals that acrolein is also detected in non-heat-treated agricultural foods, challenging the traditional assumption that it forms exclusively during thermal processing. This finding suggests that acrolein formation mechanisms are more complex than previously understood, extending to natural and endogenous pathways within agricultural products.
A: Acrolein is a significant threat to human health due to its potential to cause a wide range of diseases, including multiple sclerosis, Alzheimer's disease, other neurological disorders, cardiovascular disease, diabetes, hepatotoxicity, and nephrotoxicity through both direct and indirect toxicological mechanisms. Directly, its electrophilic nature allows it to interact with cellular nucleophiles, disrupting the functions of proteins, DNA, and RNA, leading to cellular dysfunction. Indirectly, it affects mitochondrial function, induces inflammation, alters immune responses, causes endoplasmic reticulum stress, and disrupts membrane integrity. The International Agency for Research on Cancer (IARC) has classified acrolein as a probable human carcinogen (Group 2A).