1H-Tetrazole
CAS number: 100043-29-6
1H-tetrazole is an odorless white to light-yellow crystalline powder. Mp:1 55-157 °C. When heated to decomposition it emits toxic oxides of nitrogen fumes. Can explode if exposed to shock or heat from friction or fire. The primary hazard is from blast effect where the entire load can explode instantaneously and not from flying projectiles and fragments.
Related images
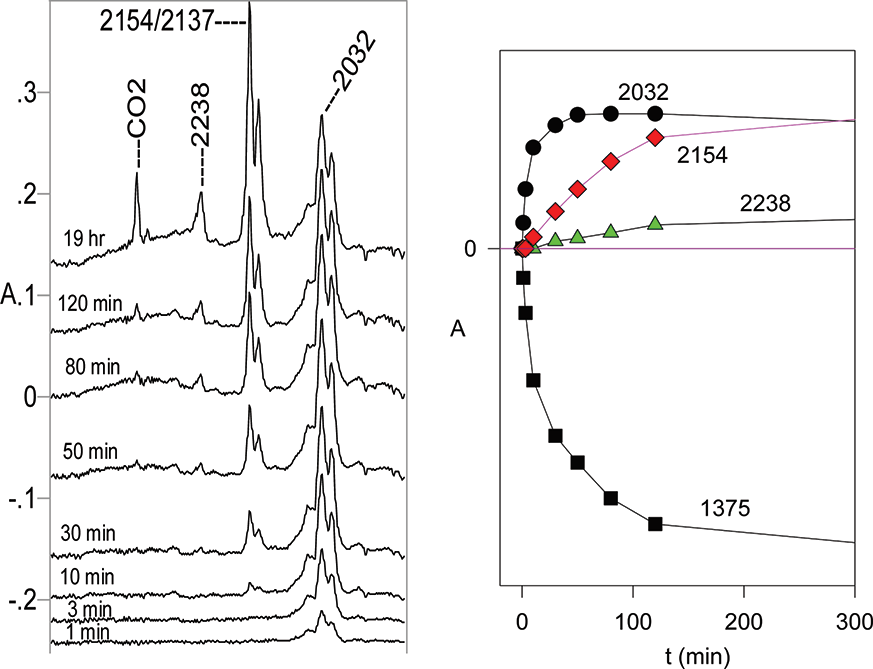
(Left) Partial IR difference spectra of tetrazole 16 at 12 K in Ar matrix at different photolysis times at 254 nm. The positive peaks are due to the photolysis products. The peaks at 2154 and 3137 cm−1 belong to the same compound, N-methyl-N′-phenylcarbodiimide 20. Abscissa 1900−2500 cm−1. (Right) Plots of IR absorbances at different wavenumbers versus photolysis time. The 1375 cm−1 band belongs to the starting material 16. Ordinates in arbitrary absorbance units.
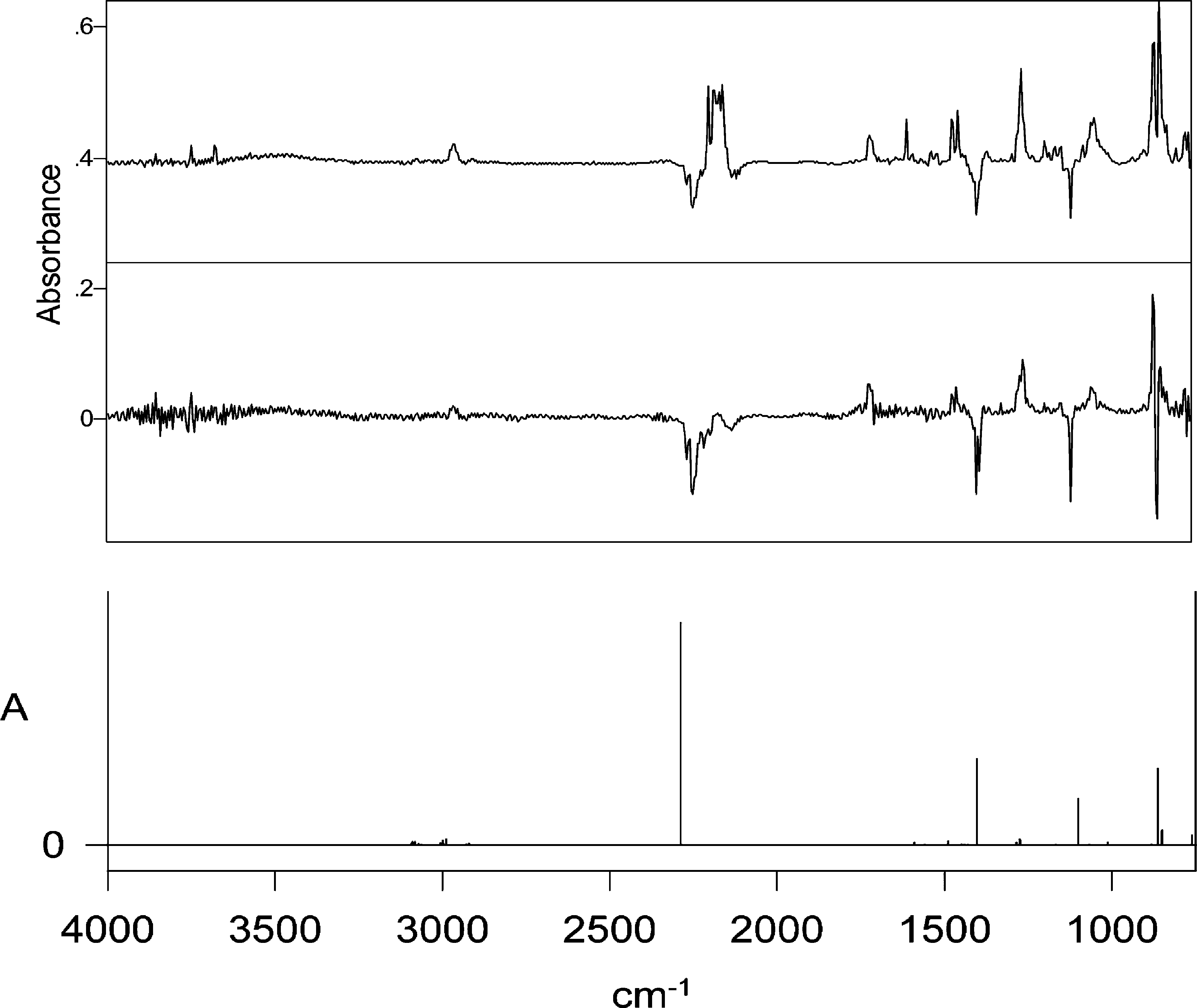
(a, bottom) Calculated IR spectrum of C-phenyl-N-(trimethylsilyl)nitrile imine 23 (B3-LYP/631G*, harmonic wave-numbers scaled by 0.9613; ordinate in arbitrary absorbance units). (b, middle) C-Phenyl-N-(trimethylsilyl)nitrile imine 23 obtained by photolysis of tetrazole 22 at 254 nm for 1 min in Ar matrix at 12 K and then destroyed by photolysis at 314 nm for 7 min, giving rise to the difference spectrum. Negative peaks are due to the nitrile imine 23: 2245, 1394, 1385, 1108, and 851 cm−1. Positive peaks are due to the formed N-phenyl-N′-(trimethylsilyl)carbodiimide 25: 2140−2175, 1590, 1466, 1451, 1256, 1150, 862, and 848 cm−1. (c, top) IR difference spectrum after photolysis of 22 for 45 min in Ar matrix at 12 K.
Related Questions and Answers
A: NaN3 serves as a nitrogen source in the synthesis of 1H-tetrazoles. It reacts with nitriles in the presence of the CoFe2O4@HPECG/Pr-SO3H$Cu(II) catalyst to form the tetrazole ring structure through a [3+2] cycloaddition reaction.