Copper sulfate pentahydrate
CAS number: 7758-99-8
Copper Sulfate Pentahydrate is the most common sulfate salt of copper. The blue, crystalline inorganic compound is a potent emetic used as an antidote for phosphorus poisoning.
Related images
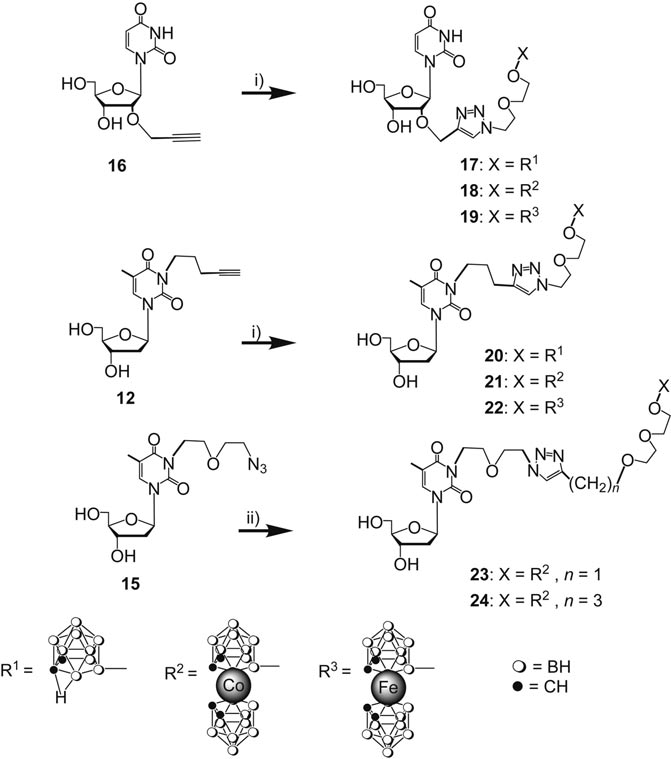
Synthesis of nucleoside borane conjugates by “chemical ligation” of nucleoside borane acceptors and borane donors: 17–24. i) 2, 5, or 6, CuSO4·5H2O/potassium ascorbate, tert-butyl alcohol/water 1:1; ii) 7 or 8, CuSO4·5H2O/potassium ascorbate, tert-butyl alcohol/water 1:1
Related Questions and Answers
A: Copper sulfate pentahydrate was effective in reducing the survival and emergence of juvenile Aedes aegypti mosquitoes. At concentrations of 300, 600, and 1200 μg/L, copper sulfate pentahydrate significantly decreased the number of larvae that survived and emerged into adult mosquitoes. The highest concentration tested (1200 μg/L) resulted in an 87.19% inhibition of emergence by day 16. This indicates that copper sulfate pentahydrate can be a viable option for vector control in water storage containers while maintaining water safety.
A: The coordination crosslinking network formed by CSP results in the formation of larger CSP particles as more rubber macromolecules are attracted to their surfaces during the curing process. This leads to a more interconnected and rigid structure, as observed in the TEM images. The size of CSP particles increases with longer curing times, indicating stronger crosslinking and immobilization of rubber macromolecules. This enhanced crosslinking also leads to a higher glass transition temperature (Tg) and a rougher tensile fractured surface, indicating stronger interfacial interactions.
Q: What is the significance of clay in the NBR/clay/copper sulfate pentahydrate (CSP) nanocomposites?
A: The clay layers in the NBR/clay/CSP nanocomposites play a crucial role in reinforcing the rubber matrix. The study shows that the presence of clay improves the dispersion and interaction with the rubber, leading to better mechanical properties. Higher clay content results in more effective reinforcement, as demonstrated by the higher tensile strength of the NBR/clay10/CSP20 nanocomposite compared to the NBR/clay5/CSP20 nanocomposite.
A: The mechanical properties of NBR/clay/CSP nanocomposites are highly dependent on the curing time. Longer curing times lead to higher crosslinking density and glass transition temperature (Tg), resulting in stronger interfacial interactions between CSP particles and the NBR matrix. The study finds that the NBR/clay10/CSP20 nanocomposite cured for 70 minutes exhibits the best mechanical properties, with a tensile strength of 37.4 MPa and an elongation at break of 354%. Further increasing the curing time does not significantly improve the tensile strength.
A: CSP serves as a coordination crosslinking agent that forms strong bonds with the nitrile groups in NBR, creating a more robust crosslinking network. This results in improved mechanical properties, such as higher tensile strength and elongation at break. The study demonstrates that increasing CSP content and optimizing curing time can significantly enhance the performance of NBR/clay nanocomposites.