Dimethyl acetylenedicarboxylate
CAS number: 762-42-5
Dimethyl acetylenedicarboxylate (DMAD) is a versatile chemical used in organic synthesis, particularly as a dienophile in Diels-Alder reactions and as a dipolarophile. DMAD is a colorless to yellow liquid at room temperature and is known for its ability to react with various nucleophiles.
Related images
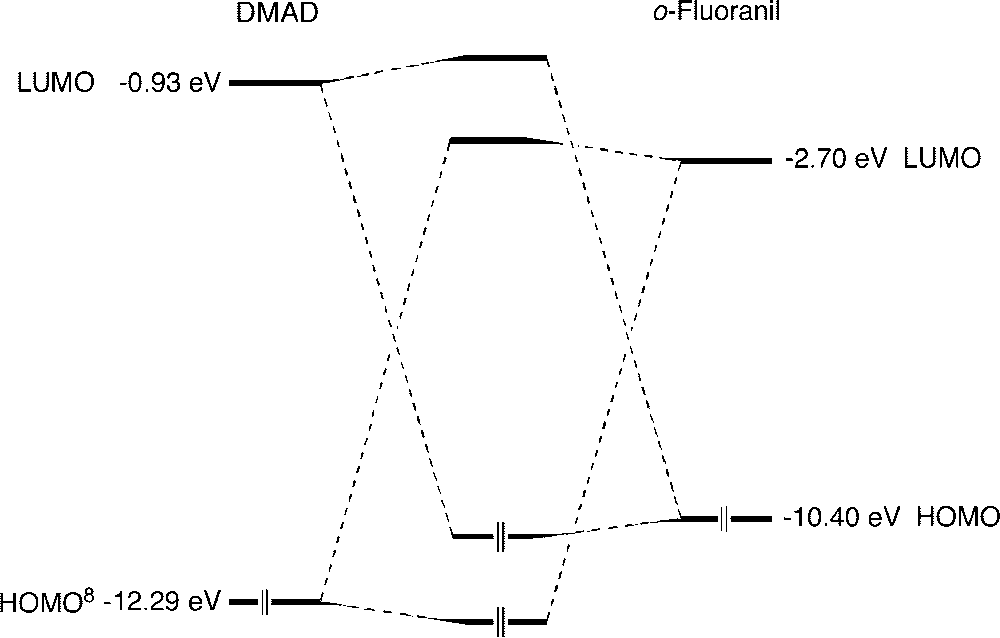
Frontier orbital interactions (AM1) in the dimethyl acetylenedicarboxylate/o-fluoranil Diels–Alder reaction.
Related Questions and Answers
A: The tautomeric behavior of the synthesized compounds is as follows:
Thiazoles (4a-d): These compounds exist in a single form without observable tautomerism. Their 1H NMR spectra show doubling of signals, indicating the presence of isomers.
Thiophenes (5c,d): These compounds exhibit tautomerism, existing in equilibrium between keto and hydroxy forms. The 1H NMR spectra of these compounds show doubling of signals, which disappears at higher temperatures (80°C and 100°C), indicating tautomerization rather than the formation of regioisomers.
Thiophenes (8a-d): These compounds do not show doubling of signals in their 1H NMR spectra, indicating that they do not undergo tautomerism. They exist in a stable planar structure stabilized by intramolecular hydrogen bonds.
Thiazoles (4a-d): These compounds exist in a single form without observable tautomerism. Their 1H NMR spectra show doubling of signals, indicating the presence of isomers.
Thiophenes (5c,d): These compounds exhibit tautomerism, existing in equilibrium between keto and hydroxy forms. The 1H NMR spectra of these compounds show doubling of signals, which disappears at higher temperatures (80°C and 100°C), indicating tautomerization rather than the formation of regioisomers.
Thiophenes (8a-d): These compounds do not show doubling of signals in their 1H NMR spectra, indicating that they do not undergo tautomerism. They exist in a stable planar structure stabilized by intramolecular hydrogen bonds.
A: The reaction of cyanothioacetamides with DMAD leads to the formation of either thiazoles or thiophenes, depending on the structure of the cyanothioacetamide and the solvent used. Specifically:
Thiazoles (4a-d): Formed as the major products when reactions are conducted in ethanol, especially with cyanothioacetamides having bulky substituents on nitrogen (e.g., cyclohexyl and isopropyl). The yields range from 30% to 63%.
Thiophenes (5c,d): Formed as major products when reactions are conducted in acetic acid. The presence of bulky substituents on nitrogen and the second thioamide group favors the formation of thiophenes. The yields range from 34% to 75%.
Influence of Solvent: Acetic acid promotes the formation of thiophenes due to the formation of an iminium intermediate, which is stabilized by intermolecular hydrogen bonds. In ethanol, thiazoles are favored.
Structural Stability: The thiophenes formed have a planar structure stabilized by intramolecular hydrogen bonds. The presence of a second thioamide group in the intermediates also influences the reaction pathway, directing the formation of specific products.
Thiazoles (4a-d): Formed as the major products when reactions are conducted in ethanol, especially with cyanothioacetamides having bulky substituents on nitrogen (e.g., cyclohexyl and isopropyl). The yields range from 30% to 63%.
Thiophenes (5c,d): Formed as major products when reactions are conducted in acetic acid. The presence of bulky substituents on nitrogen and the second thioamide group favors the formation of thiophenes. The yields range from 34% to 75%.
Influence of Solvent: Acetic acid promotes the formation of thiophenes due to the formation of an iminium intermediate, which is stabilized by intermolecular hydrogen bonds. In ethanol, thiazoles are favored.
Structural Stability: The thiophenes formed have a planar structure stabilized by intramolecular hydrogen bonds. The presence of a second thioamide group in the intermediates also influences the reaction pathway, directing the formation of specific products.