beta-Cyclodextrin
CAS number: 7585-39-9
Beta-cyclodextrin is a cyclodextrin composed of seven alpha-(1->4) linked D-glucopyranose units.
Related images
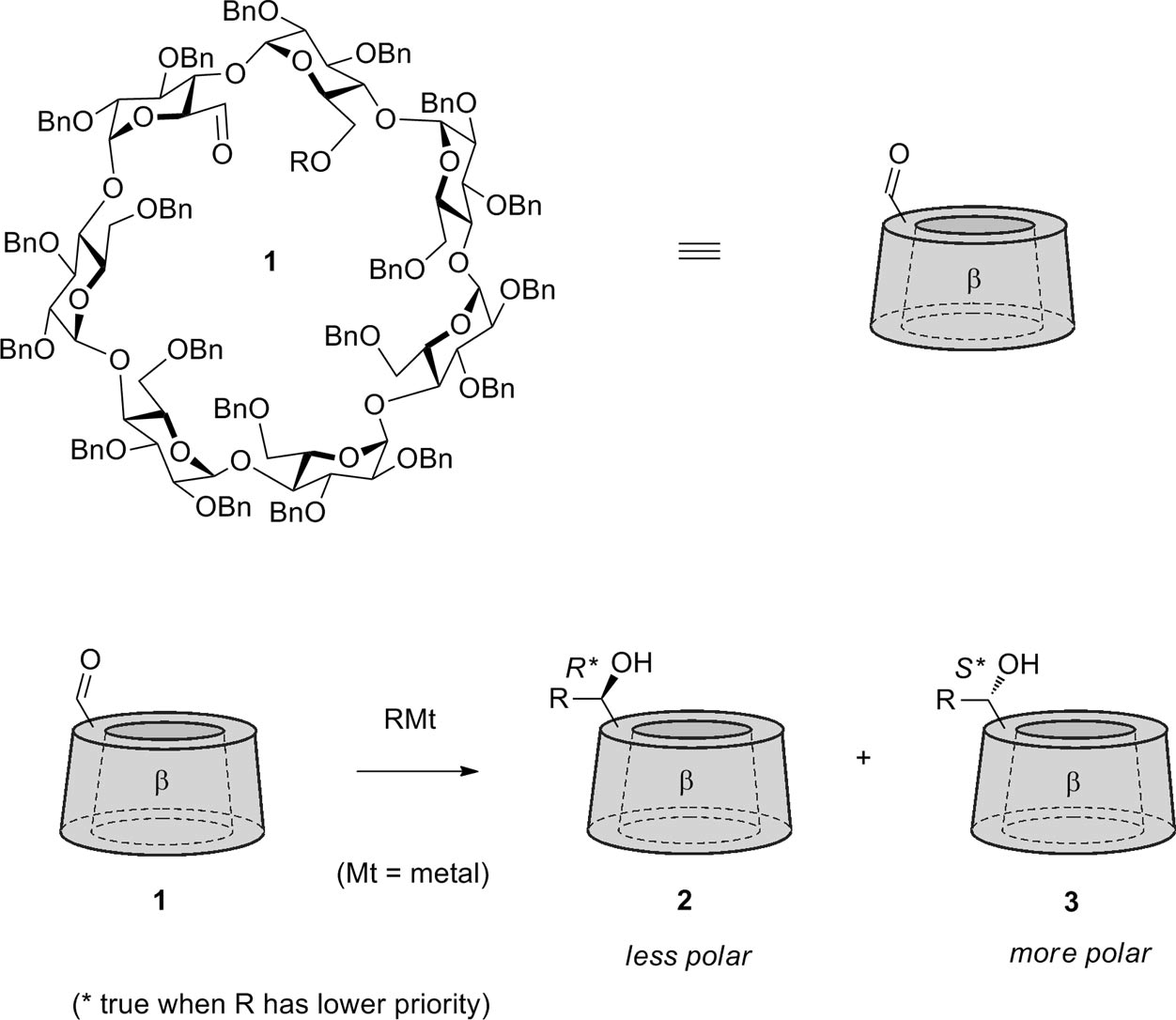
Benzylated β-cyclodextrin-aldehyde and its reaction with organometallic reagents. This leads to two stereoisomers with rather different polarity.
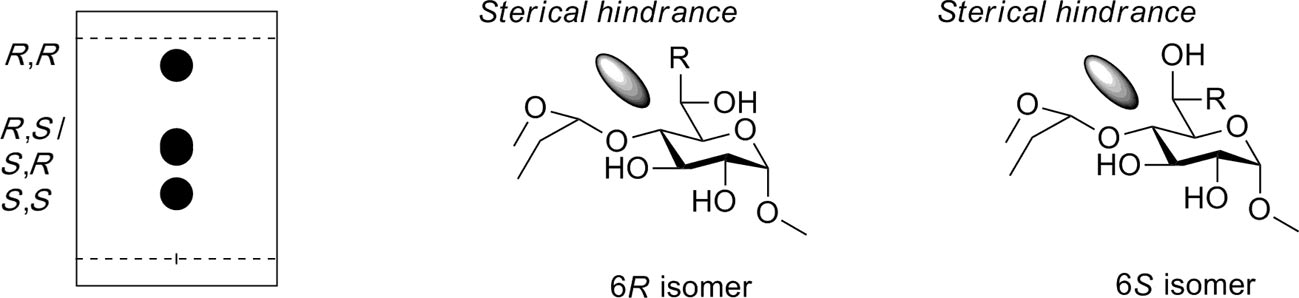
TLC plate showing the different polarities of diastereoisomers 6A,D-di-C-allyl-substituted nonadecaned-O-benzylated β-cyclodextrin in pentane/ethyl acetate 4:1 eluent (left). The figure shows how steric hindrance of the subsequent glucose residue prevents the C-6 substituent from entering the tg conformation, thus forcing each diastereomer to adopt the conformer shown (right).
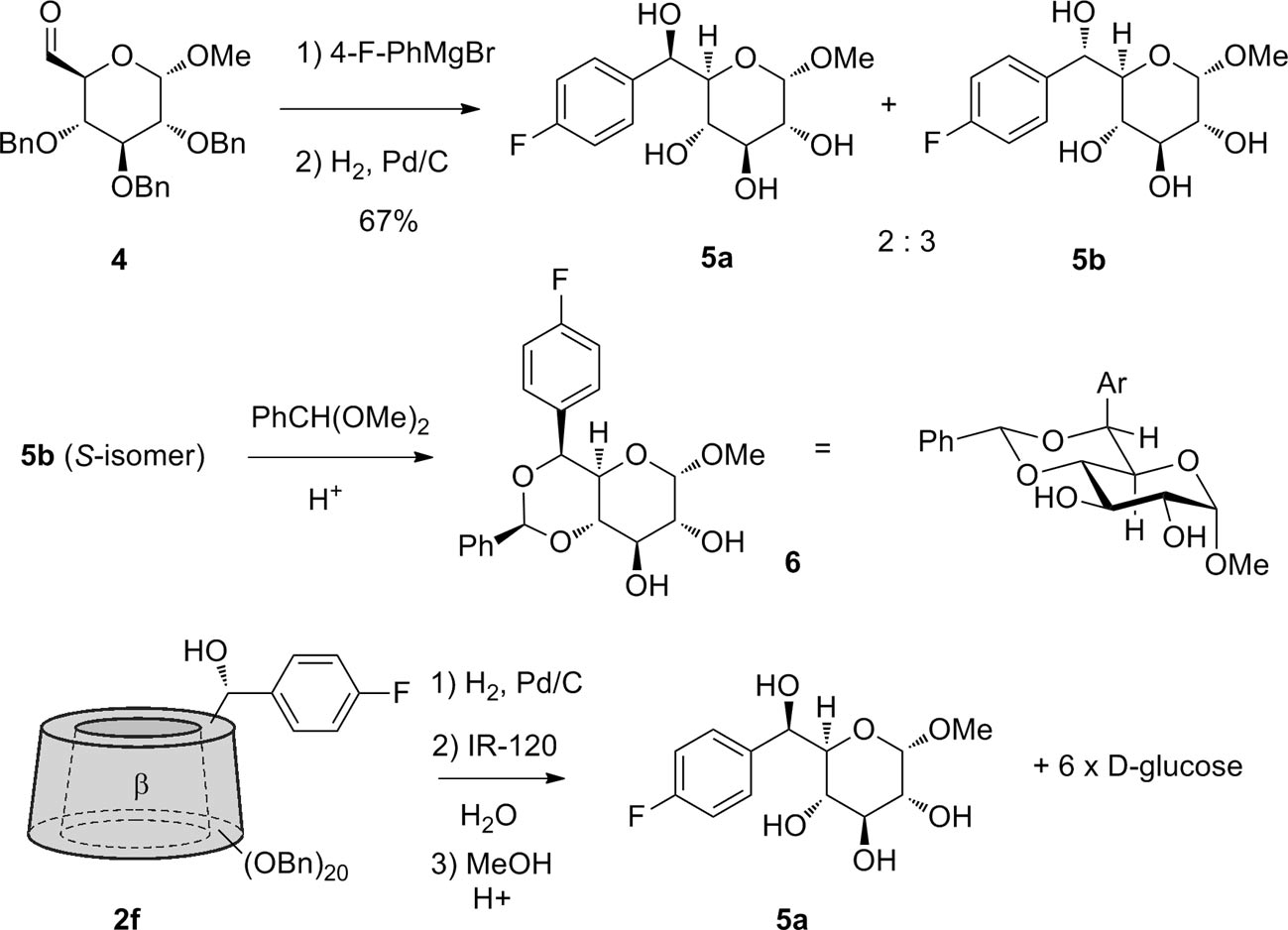
Two reference monosaccharide derivatives 5a and 5b were prepared to determine the stereochemistry at the C-6 position. The major isomer 5b obtained by addition of (4-fluorophenyl)magnesium bromide to glucose-6-aldehyde 4 was converted to the benzylidene derivative 6. This compound has a small J5,6 and only conforms to the S-stereochemistry. The β-cyclodextrin derivative 2f was hydrolyzed and converted to the methyl glycoside to give the minor R-isomer 5a.
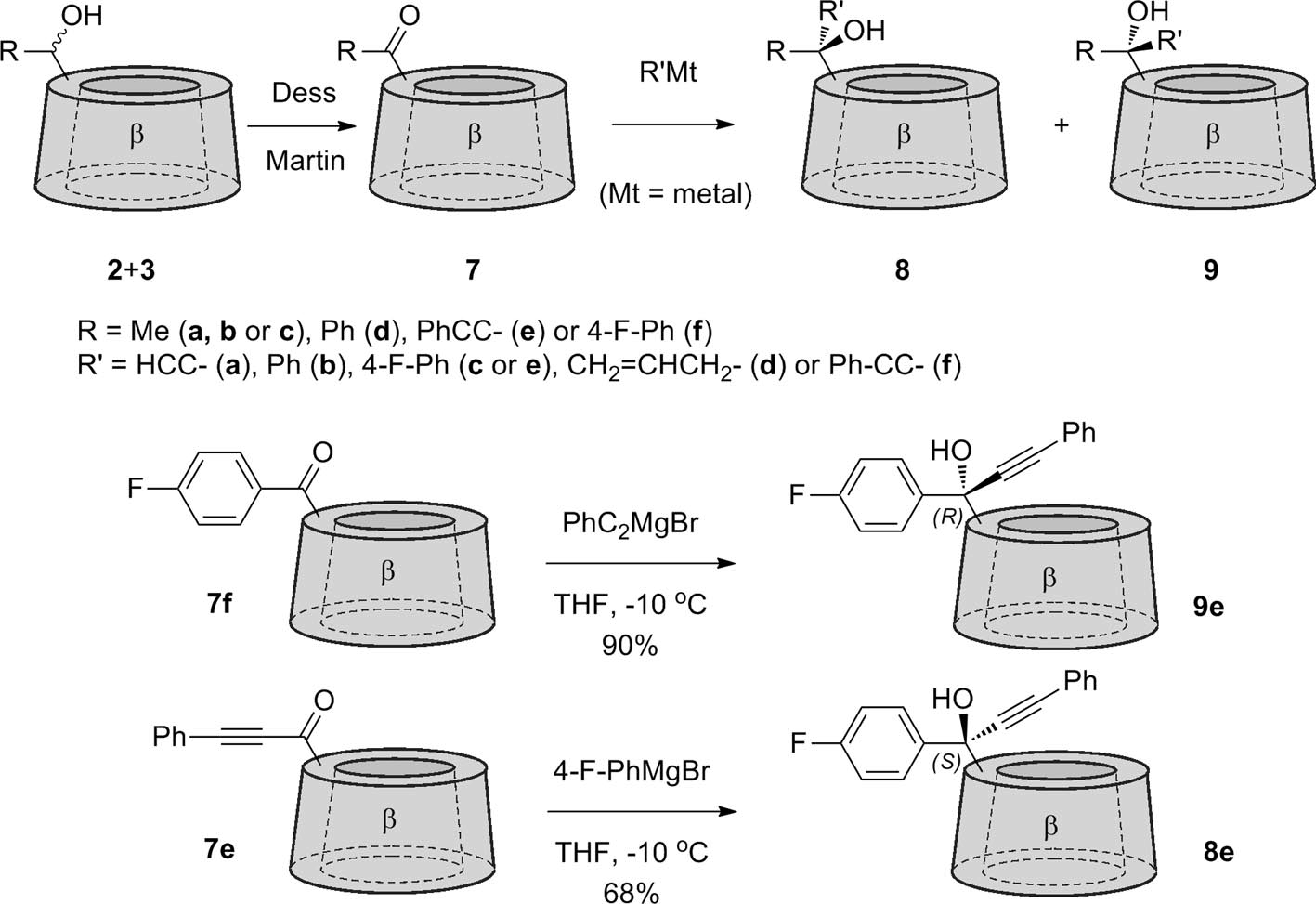
Oxidation of secondary alcohols to the β-cyclodextrin ketone 4, 7e and 7f followed by reaction with organometallic reagents to give tertiary alcohols.
Related Questions and Answers
No related questions yet