L-Arginine
CAS number: 74-79-3
L-arginine is an amino acid that helps build protein and acts as a vasodilator, opening blood vessels. It is generally safe.
Related images
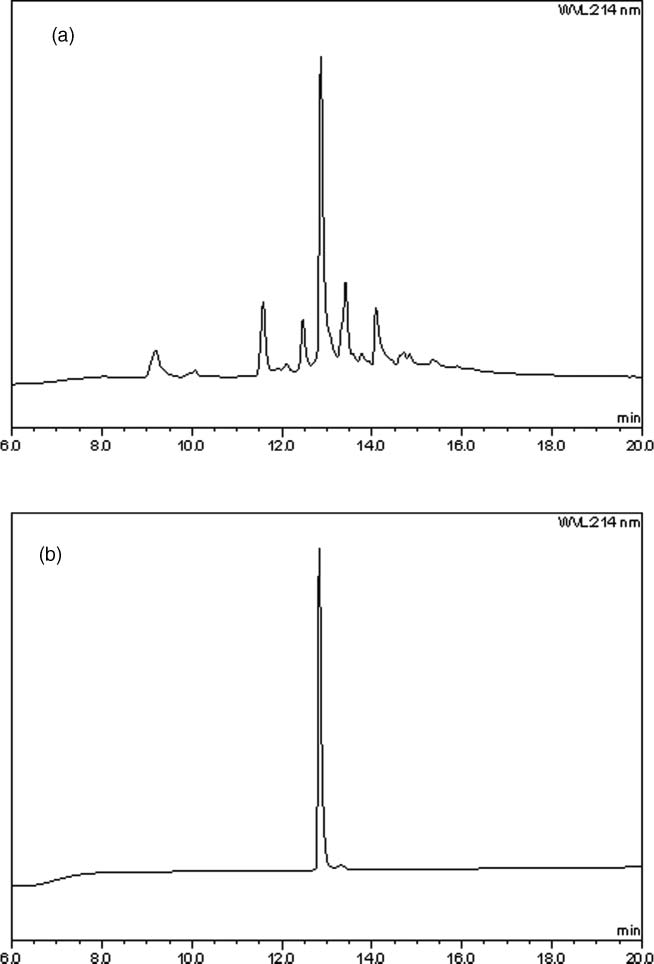
(a) HPLC spectrum of crude arginine 3 after solid phase assembly and two-step deprotection. Conditions: Same as Figure 3. (b) HPLC spectrum of arginine 3 after single-step final purification. Conditions: Same as Figure 3.
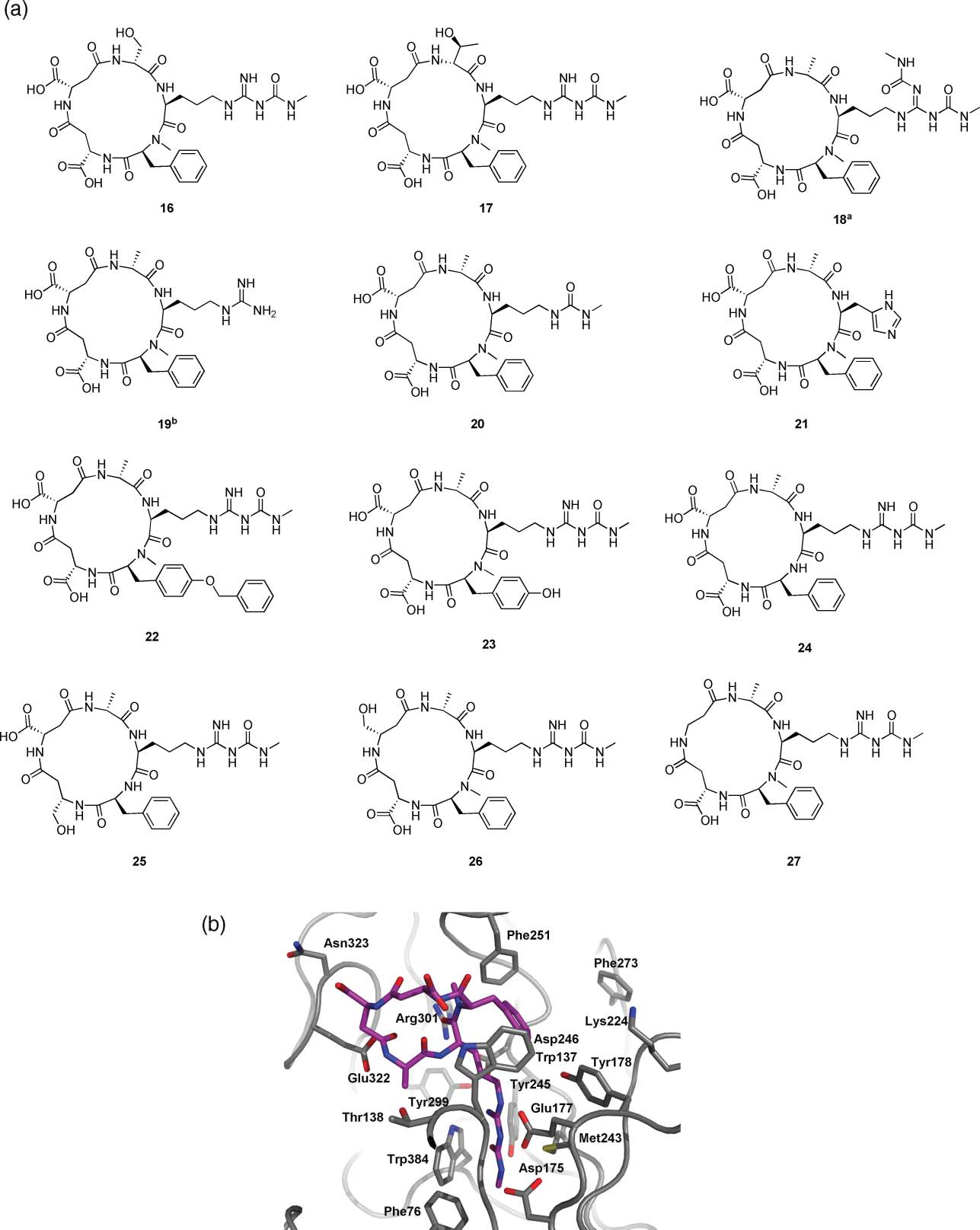
(a) Compounds investigated in this study. Byproducts in the synthesis of 3. Intermediates in the synthesis of 3. (b) X-ray structure of arginine 3 in complex with Af ChiB1, showing the key residues for SAR formation (model extracted from previously published complex 22a, PDB entry 1W9V).
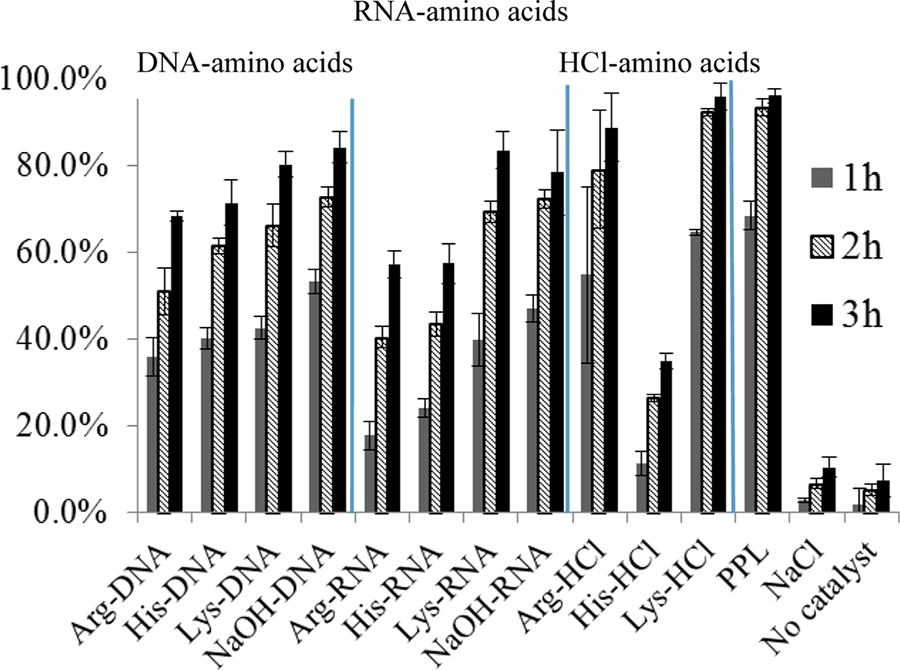
When the nucleotides were neutralized to pH 7.0 with NaOH or basic amino acids (L-lysine, L-arginine, or L-histidine), the reaction rate was significantly accelerated and the yield was comparable to that of porcine pancreatic lipase (PPL, one of the best enzymes for the Knoevenagel condensation reaction) as well as pH-neutral amino acid salts Arg•HCl and Lys•HCl.
Related Questions and Answers
Q: Why are arginine and arginine-containing di- and tripeptides significant in H/D exchange reactions?
A: Arginine and its di- and tripeptides are key subjects for understanding H/D exchange mechanisms in the gas phase. Arginine and arginine-containing tripeptides exhibit three H/D exchanges, whereas dipeptides show a single exchange. The high proton affinity of the guanidine group in arginine dictates its protonation site, which is crucial for these reactions.