Diisopropylethylamine
CAS number: 7087-68-5
N,N-Diisopropylethylamine (DIPEA) is a tertiary amino compound. It is a colorless liquid and is also known as Hunig's base.
Related images
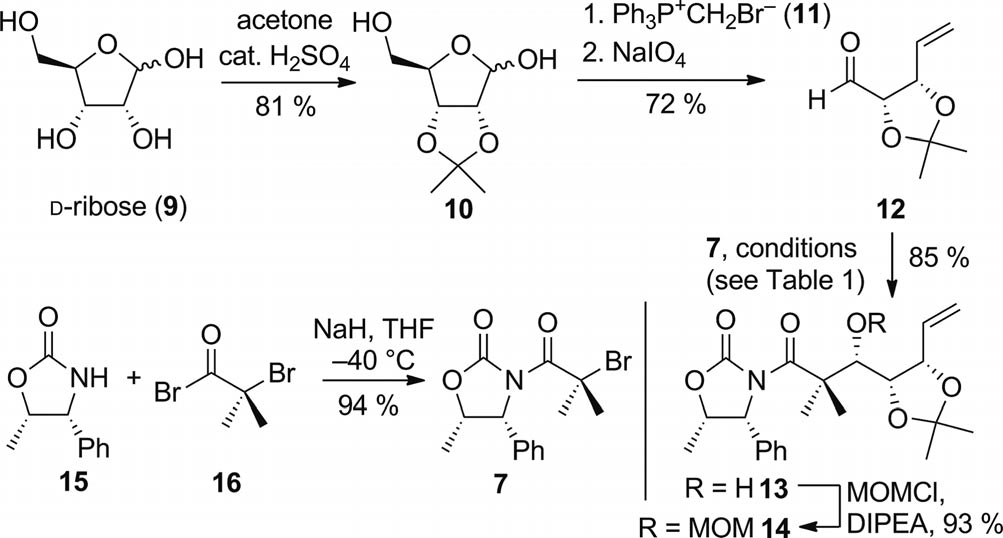
Preparation of alkene 14 (DIPEA = N,N-diisopropyl-ethylamine).
![Peptoid-synthesis-inspired modular assembly of azaxylylene precursors and their cyclizations. [a] Acetal hydrolysis step is required only in the a (i.e. benzaldehyde) series. [b] Major diastereomer is shown, the minor hydroxy epimer (not shown) is denoted with a prime, 16’a etc. Bn=benzyl, DIPEA=diisopropylethylamine, PTS=pyridinium para-tolue-nesulfonate.](http://www.wlxkc.cn/picture/3047941_09.png)
Peptoid-synthesis-inspired modular assembly of azaxylylene precursors and their cyclizations. [a] Acetal hydrolysis step is required only in the a (i.e. benzaldehyde) series. [b] Major diastereomer is shown, the minor hydroxy epimer (not shown) is denoted with a prime, 16’a etc. Bn=benzyl, DIPEA=diisopropylethylamine, PTS=pyridinium para-tolue-nesulfonate.
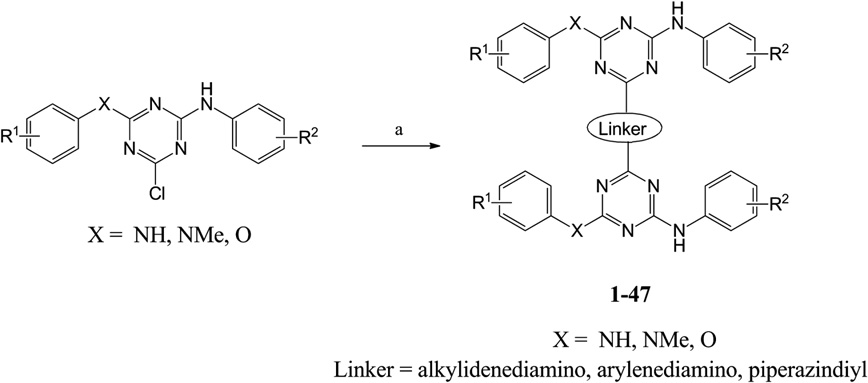
Synthesis of triazine dimers 1–47. Reagents and conditions: (a) diaminoalkanes/diaminobenzene/piperazine, DIPEA, dry dioxane, 110 °C.
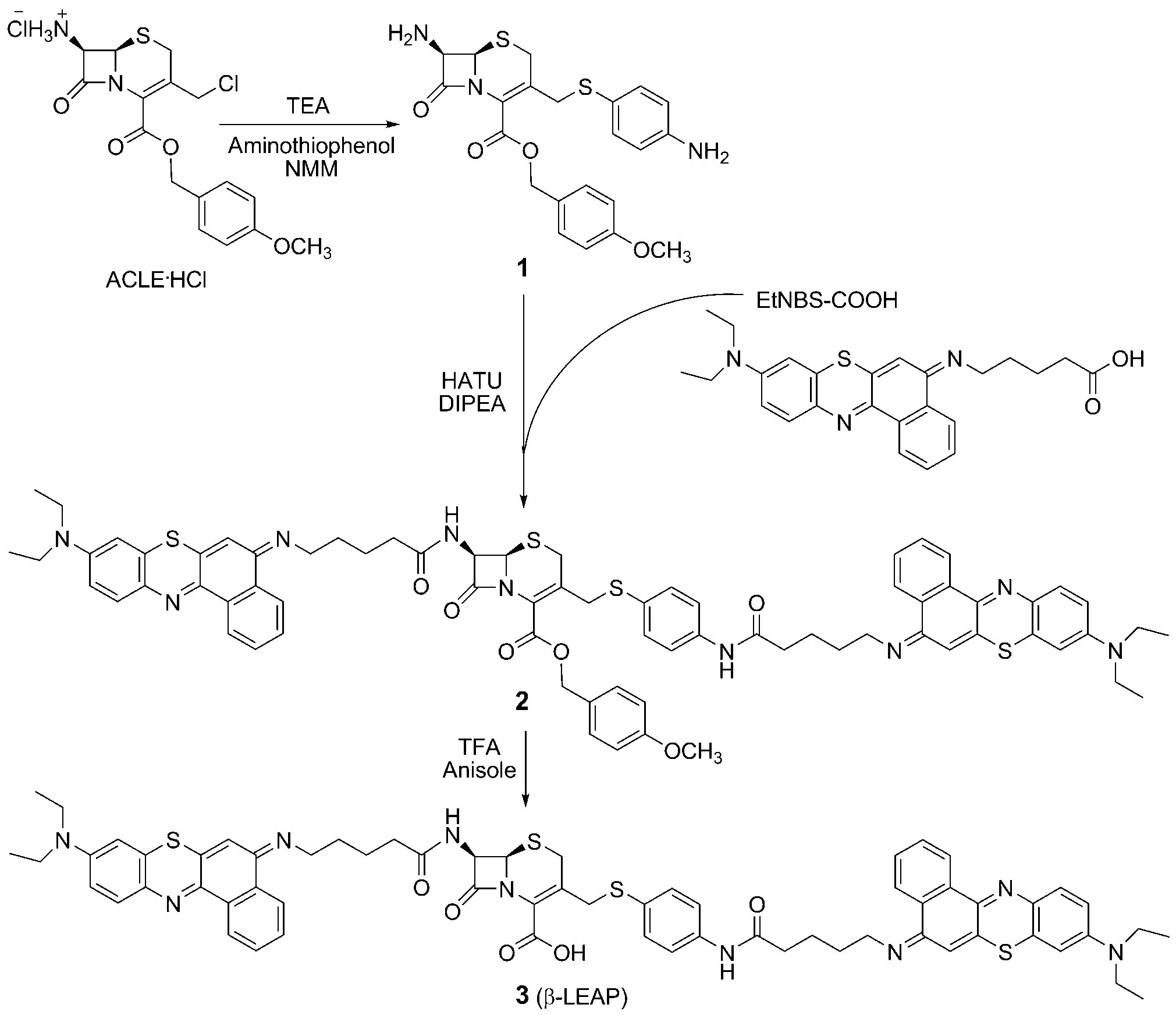
Synthesis of β-LEAP. DIPEA=N,N’-diisopropylethylamine, NMM=4-methylmorpholine, HATU=O-(7-azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate, TEA=triethylamine, TFA=trifluoroacetic acid.
Related Questions and Answers
No related questions yet