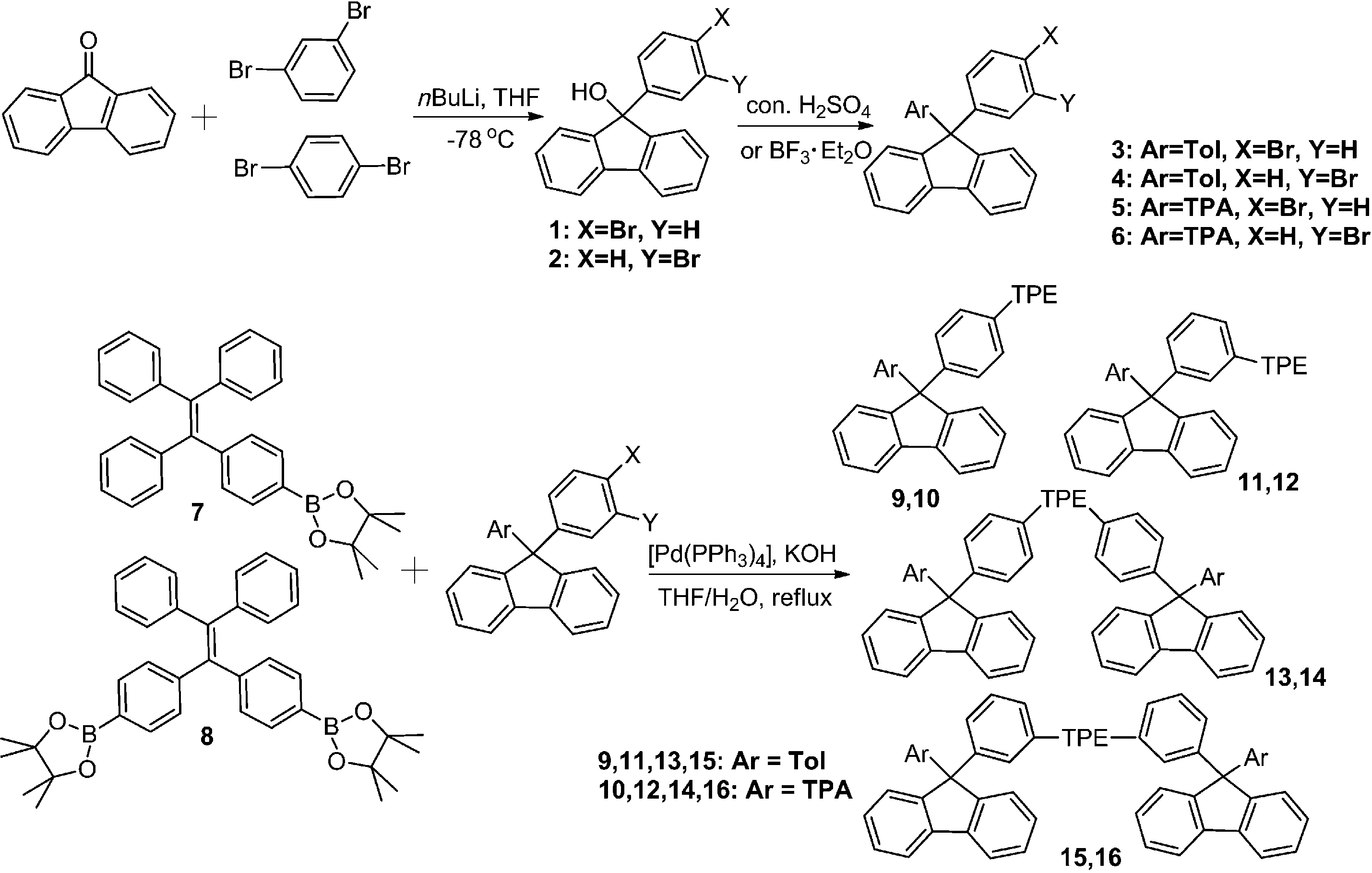
Tetraphenylethylene
CAS number: 632-51-9
Tetraphenylethylene (TPE) is an organic compound which known for its propeller-shaped structure with four phenyl rings attached to a central double bond, which are twisted out of plane.
Related images
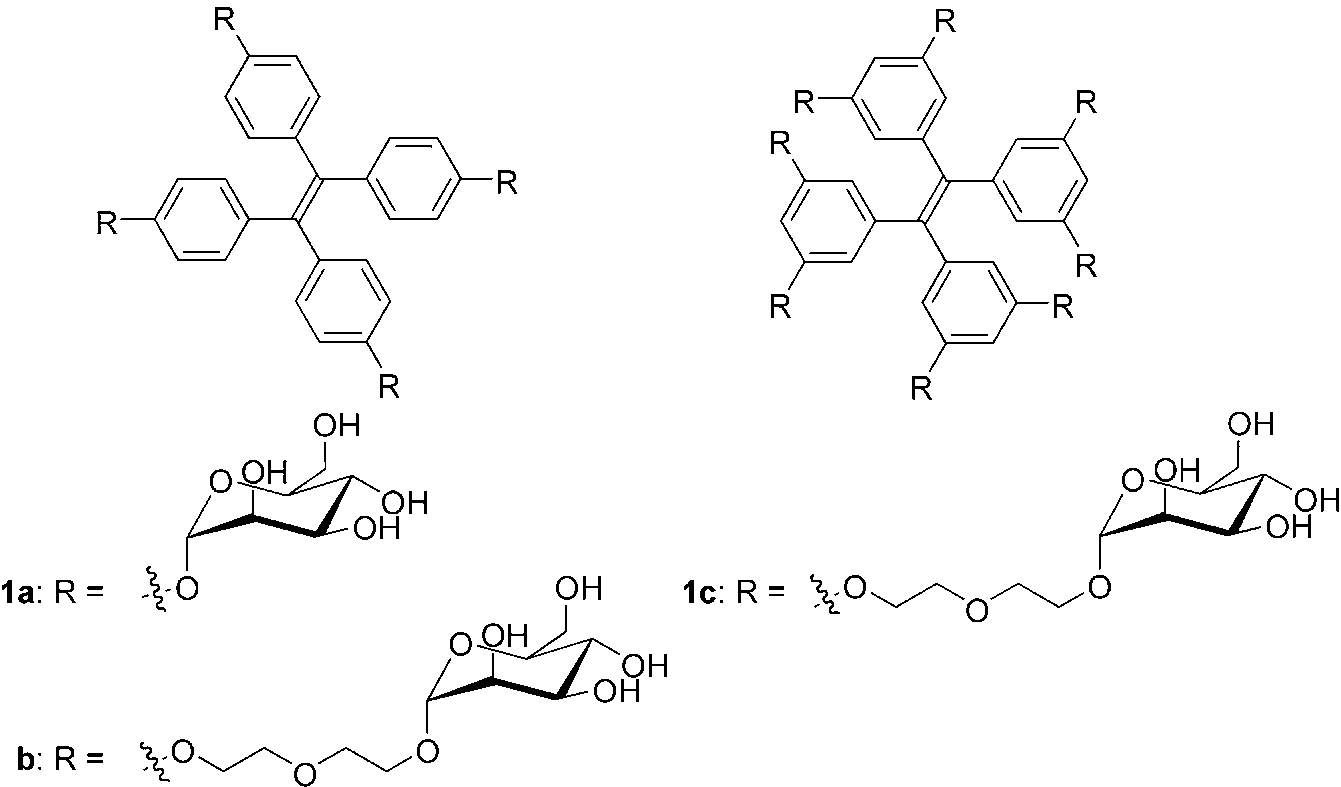
Chemical structures of mannose-substituted tetraphenylethylenes (TPEs), 1a–c.
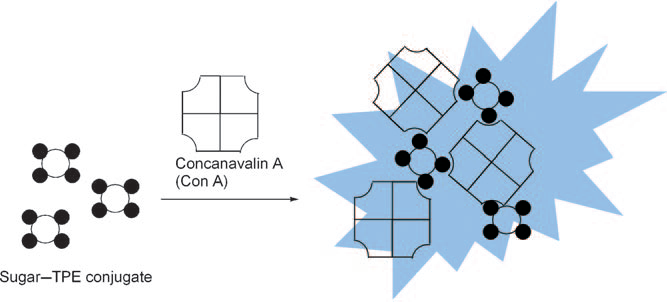
Schematic representation of a “turn-on” fluorescent sensor for Con A using a sugar-modified tetraphenylethene (TPE) based on an aggregation-induced emission (AIE).
![Plots of the fluorescence quantum yields determined in THF/H2O solutions by using 9,10-diphenylanthracene (F=90% in cyclohexane) as the internal standard versus the fraction of water in the solvent. Insets: photo- graphs of the TPE–Ar luminogens in the THF/water mixture (fw =99%) taken under the illumination of a 365 nm UV lamp. Excitation wavelengths [nm]: 320 for TPE–ptol and TPE–mtol, and 310 for the other six fluorophores.](http://www.wlxkc.cn/picture/3386517_12.png)
Plots of the fluorescence quantum yields determined in THF/H2O solutions by using 9,10-diphenylanthracene (F=90% in cyclohexane) as the internal standard versus the fraction of water in the solvent. Insets: photo- graphs of the TPE–Ar luminogens in the THF/water mixture (fw =99%) taken under the illumination of a 365 nm UV lamp. Excitation wavelengths [nm]: 320 for TPE–ptol and TPE–mtol, and 310 for the other six fluorophores.
![PL spectra of a) TPE–pTPA and b) TPE–mTPA in THF/H2O mixtures with different water fractions. Concentration [mM]: 10; excitation wavelength [nm]: 310.](http://www.wlxkc.cn/picture/3386517_14.png)
PL spectra of a) TPE–pTPA and b) TPE–mTPA in THF/H2O mixtures with different water fractions. Concentration [mM]: 10; excitation wavelength [nm]: 310.
![The PL spectra of the films of the TPE–Ar luminogens. The thin films were spin-coated onto ITO glass from dilute THF solutions with concentrations of 1 mgmLÀ1. Excitation wavelengths [nm]: 320 for TPE–2pTPA and TPE–2mTPA, 350 for TPE–2ptol and 340 for the rest of the fluorophores.](http://www.wlxkc.cn/picture/3386517_15.png)
The PL spectra of the films of the TPE–Ar luminogens. The thin films were spin-coated onto ITO glass from dilute THF solutions with concentrations of 1 mgmLÀ1. Excitation wavelengths [nm]: 320 for TPE–2pTPA and TPE–2mTPA, 350 for TPE–2ptol and 340 for the rest of the fluorophores.

Calculated molecular orbital amplitude plots of HOMO and LUMO levels of the TPE–Ar luminogens.
Related Questions and Answers
No related questions yet