Kinetin
CAS number: 525-79-1
Kinetin is a cytokinin, or adenine derivative, found in various plants and human cells.
Related images
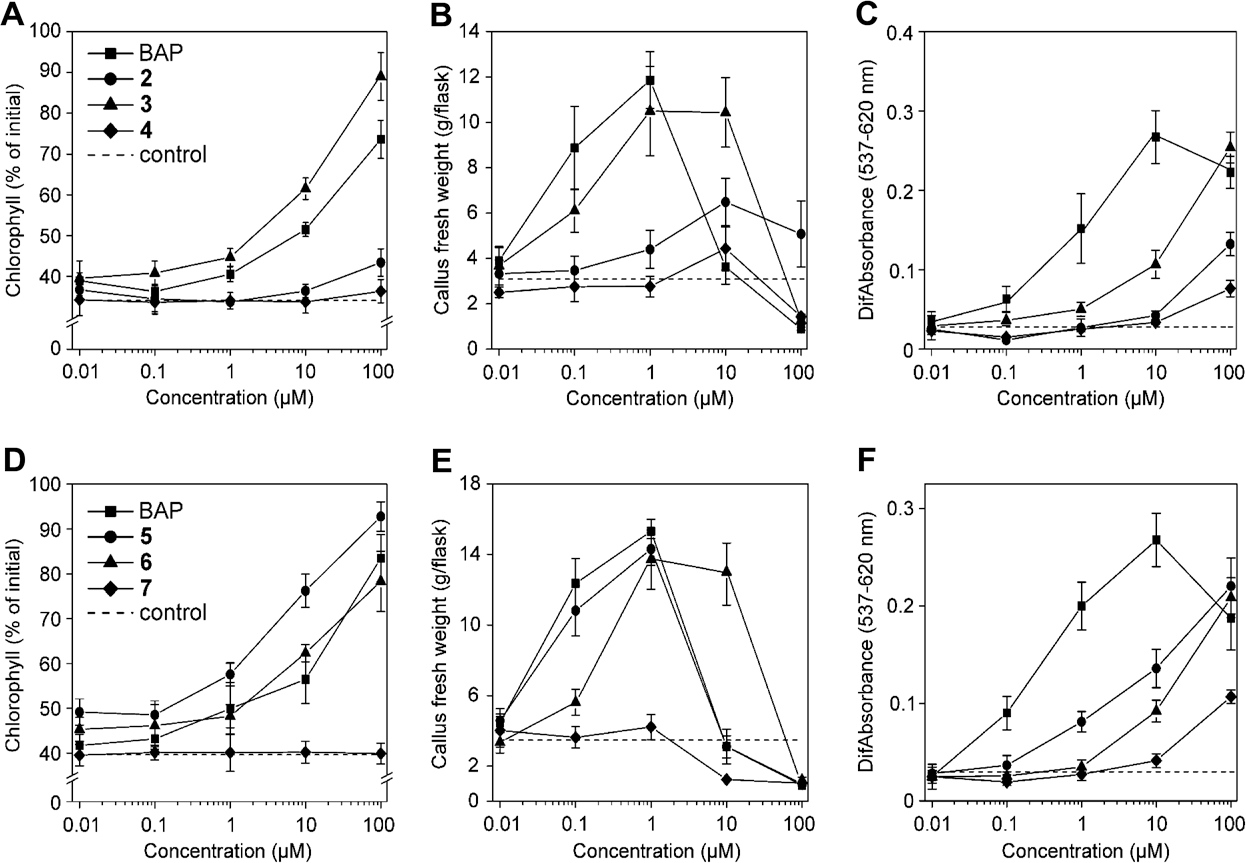
The Influence of hydroxy or methoxy group position in the benzyl ring of 6-benzyl-9-tetrahydropyran-2-yl-purines on their biological activity in classical cytokinin bioassays. Comparison of the impact of hydroxy (A–C) and methoxy (D–F) groups substituted in ortho- (circles), meta- (triangles), and para- (diamonds) positions, respectively, on: the retention of chlorophyll in excised wheat leaves (A, D); the stimulation of cytokinin-dependent tobacco callus growth (B, E); and the dark induction of betacyanin synthesis in Amaranthus cotyledons (C, F).
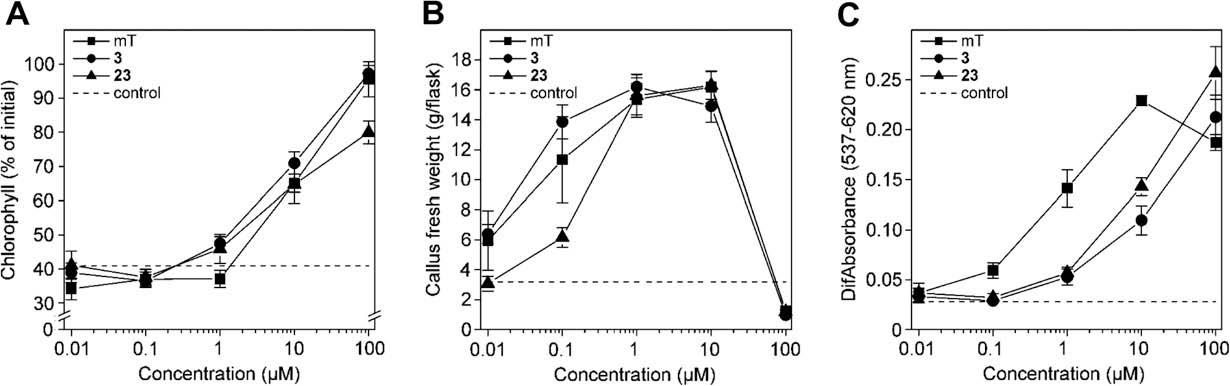
The Influence of 9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-yl groups on the biological activity of 6-benzyl-(3-hydroxybenzylamino)purine in classical cytokinin bioassays. (A) The effect on chlorophyll retention in excised wheat leaf tips; (B) the growth of cytokinin-dependent tobacco callus; (C) the effect on dark betacyanin synthesis in Amaranthus caudatus cotyledon-hypocotyl explants.
Related Questions and Answers
A: Kinetin was found to be the least active cytokinin among those tested. It did not induce the development of new shoots, with the original shoots developing further but without significant new shoot formation. Therefore, KIN is not recommended for in vitro shoot multiplication of Húsvéti Rozmaring apple scion due to its limited effectiveness.