Dichloromethane-d2
CAS number: 1665-00-5
Dichloromethane-d2, also known as methylene chloride-d2 or dideuteromethylenechloride, is a deuterated form of the solvent dichloromethane (CH2Cl2).
Related images
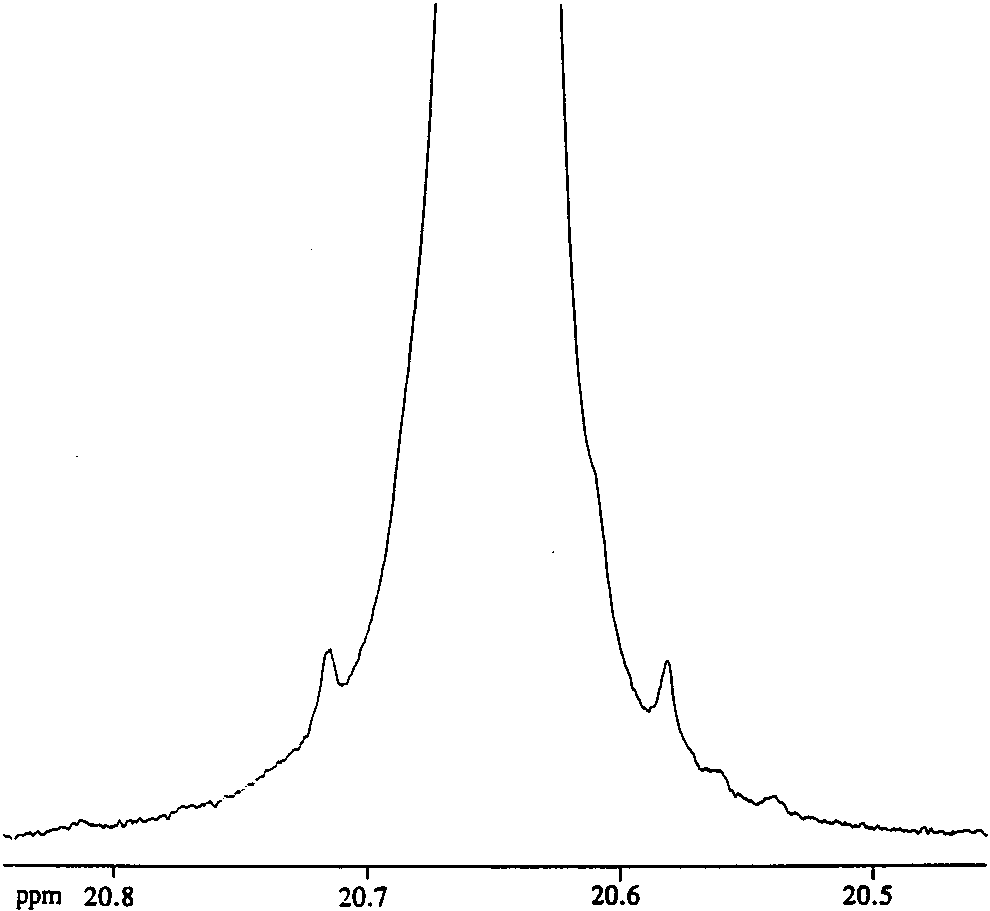
Zoom-in of the nitroxyl protons in the 1H NMR spectrum of 3a (CD2Cl2, 400 MHz, 20 °C).
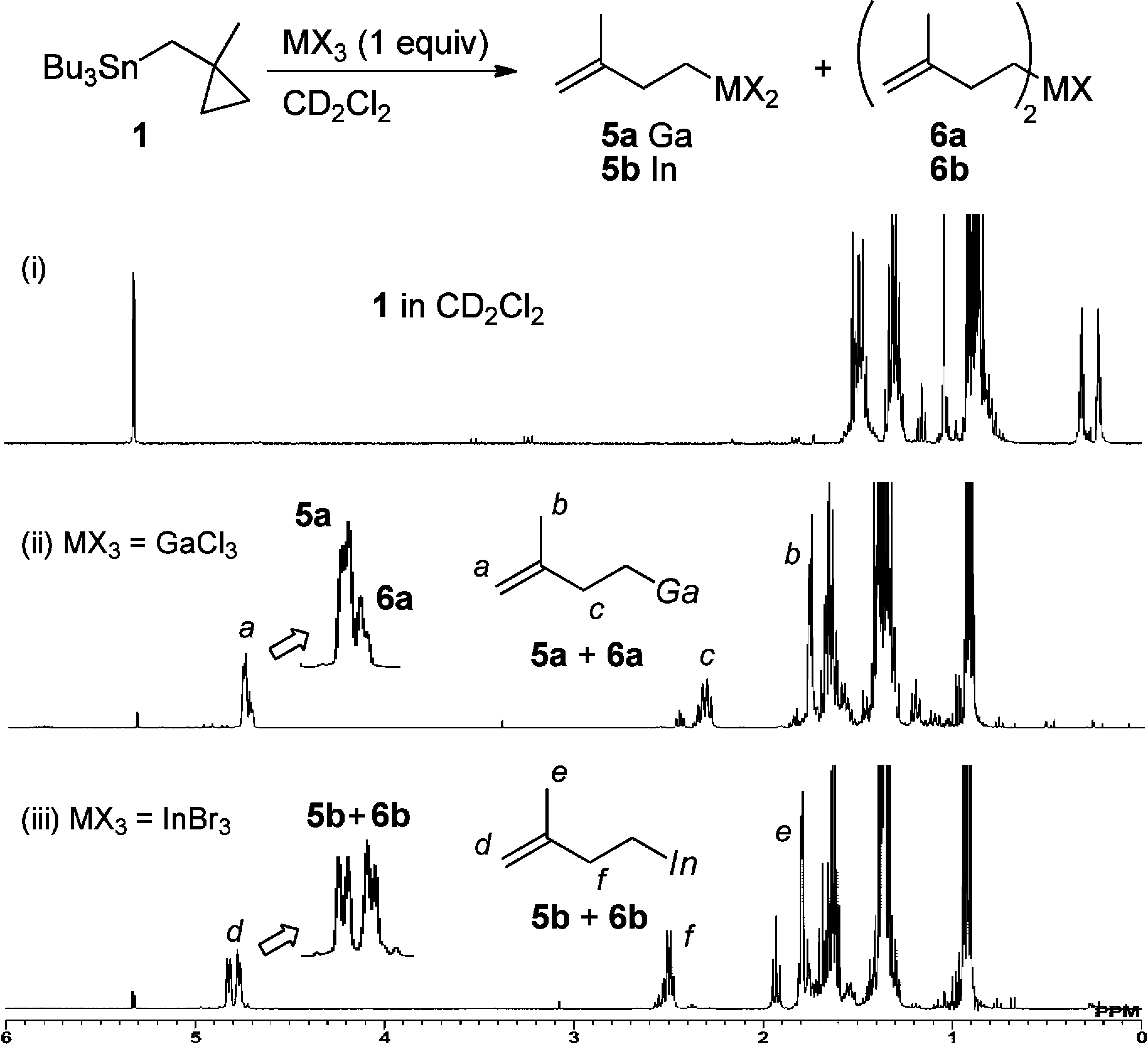
1H NMR spectra of (i) 1, (ii) the mixture of GaCl3 and 1, and (iii) the mixture of InBr3 and 1 in CD2Cl2.
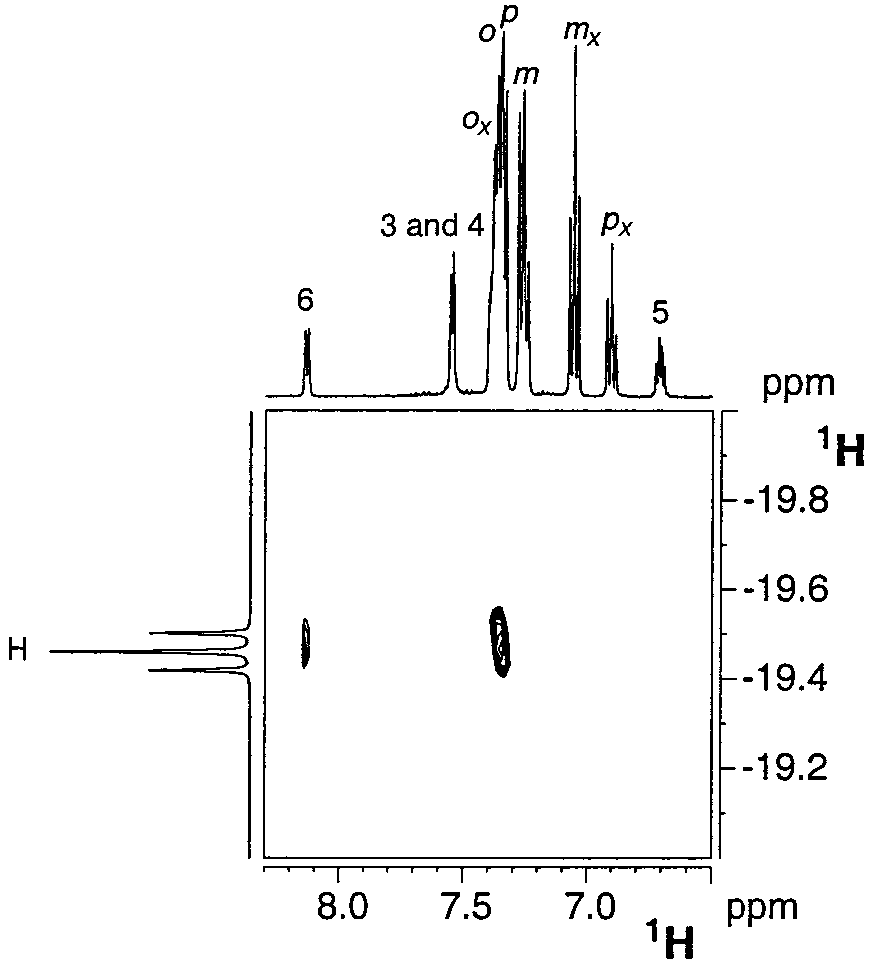
Section of the 1H-NOESY NMR spectrum of complex 1d recorded at 400.13 MHz in methylene chloride-d2 (298 K) showing the selective intramolecular interactions of the hydrides with protons H-6 and H-o.
Related Questions and Answers
A: The dynamic equilibrium of ligands in colloidal metal chalcogenide QDs is significantly affected by solvent polarity and viscosity. Aliphatic and more polar solvents enhance ligand solvation and their displacement from the QD surface, while aromatic solvents lead to broader NMR resonances and slower ligand diffusion. Conversely, aliphatic solvents such as dichloromethane-d2, chloroform-d, and hexane-d14 produced relatively narrow resonances.