Scandium triflate
CAS number: 144026-79-9
Scandium triflate, also known as scandium(III) trifluoromethanesulfonate, is a highly effective and versatile Lewis acid catalyst used in organic chemistry, particularly for Friedel-Crafts reactions, Diels-Alder reactions, and other carbon-carbon bond-forming reactions.
Related images
Related Questions and Answers
Q: What is the significance of the hydrated scandium triflate in achieving high diastereoselectivity?
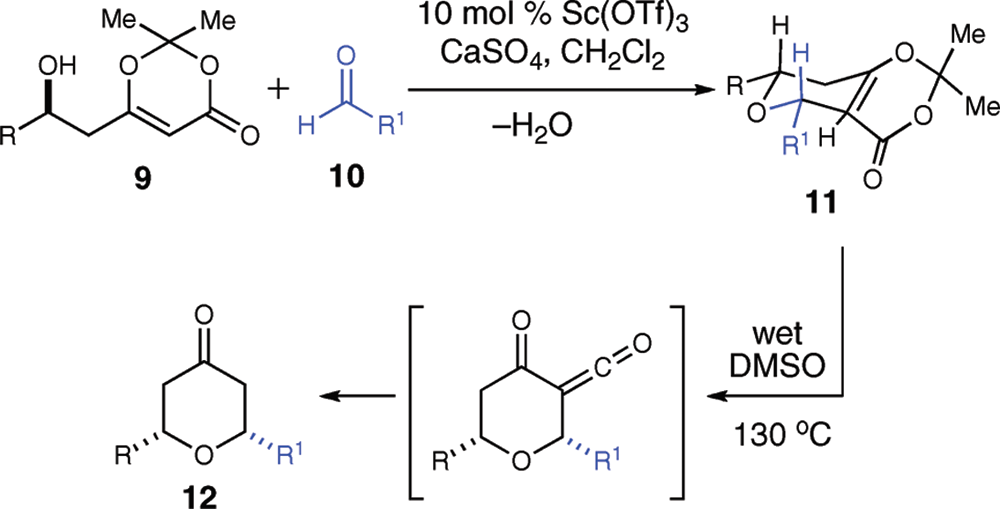
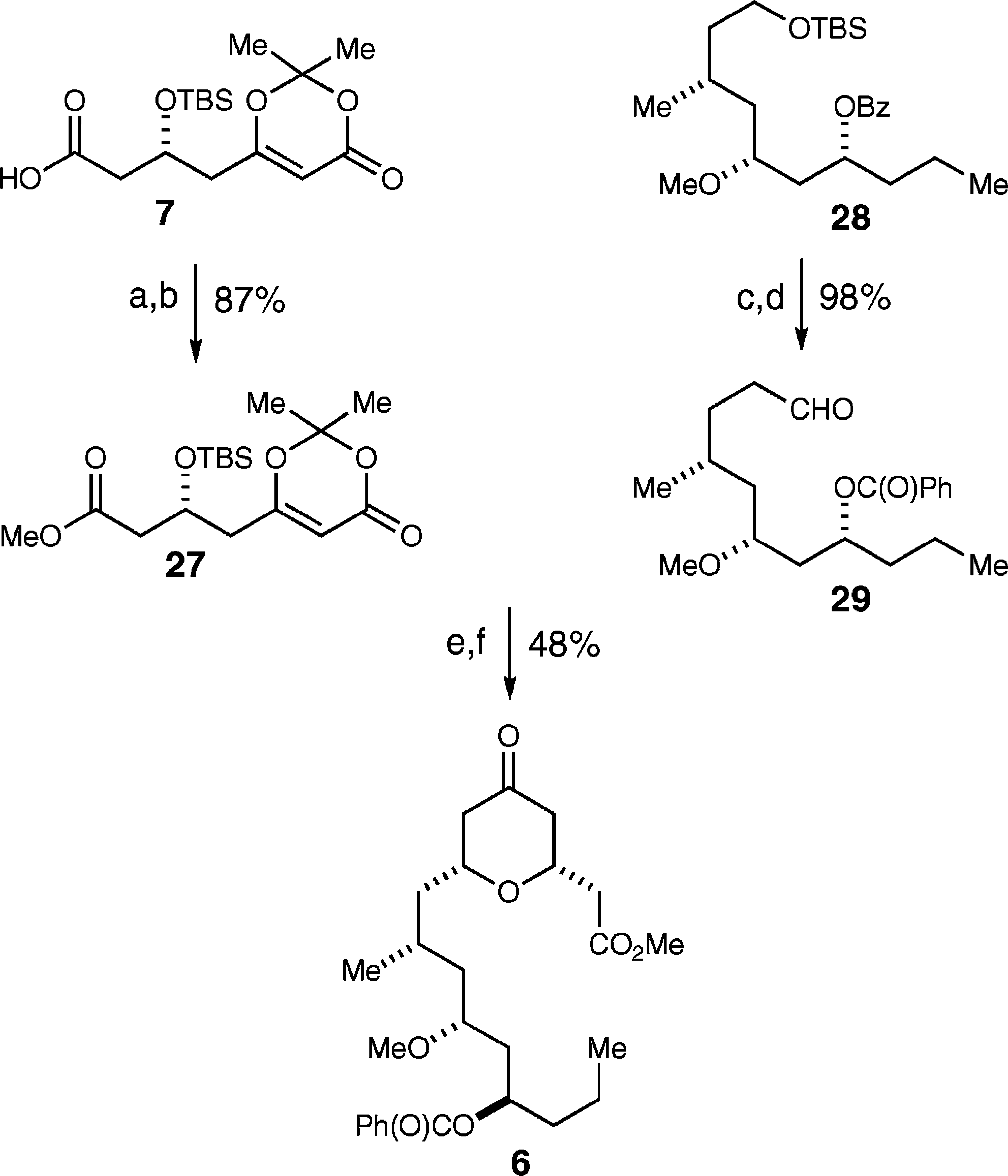
A: The hydrated form of scandium triflate (Sc(OTf)₃) is essential for achieving high diastereoselectivity in the allylation reaction. The addition of water to the anhydrous catalyst improves its performance by introducing additional steric hindrances, which further enhance the difference in reactivity between the chelate intermediates. This leads to a higher yield and better diastereoselectivity (up to 94:6) compared to using the anhydrous form of the catalyst.
A: Scandium triflate (Sc(OTf)₃) plays a crucial role as a Lewis acid catalyst in the diastereoselective allylation of (S)-3-(methoxymethyl)hexanal. The hydrated form of scandium triflate was found to be essential for achieving high 1,3-anti-diastereoselectivity (d.r. 94:6), while the anhydrous form resulted in lower diastereoselectivity (d.r. 76:24). The addition of water to the anhydrous catalyst improved its performance, leading to better diastereoselectivity and yield.