1,4-Dioxane
CAS number: 123-91-1
1,4-Dioxane is a likely human carcinogen and has been found in groundwater at sites throughout the United States. 1,4-Dioxane is a clear liquid that easily dissolves in water. It is used primarily as a solvent in the manufacture of chemicals and as a laboratory reagent.
Related images
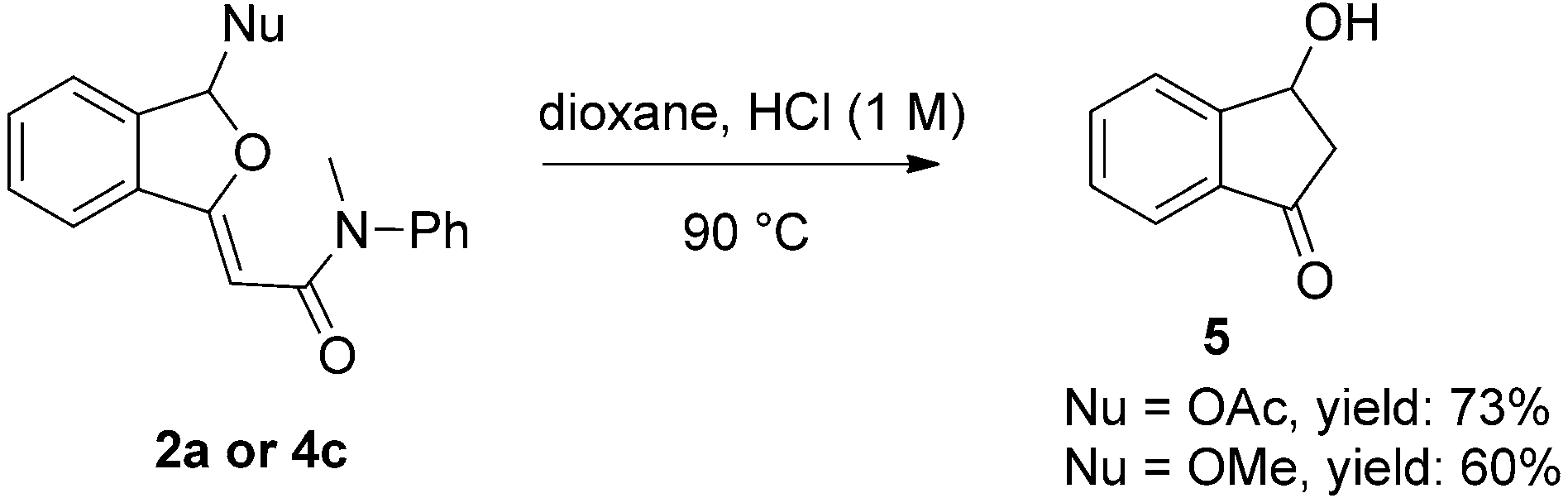
![O-Arylation of 3-phenylpropanol (1a) and phenol (1ab) by triarylsulfonium triflates in the presence of CsOH.[a] [a] Reaction conditions: 1a or 1ab (0.2 mmol), 2 (0.3 mmol), CsOH (0.3 mmol), 1,4-dioxane (2 mL), 508C, N2, 24 h. Isolated yield. [b] Toluene (2 mL) as solvent and at 808C. [c] 808C. [d] 0.4 mmol scale (1ab).](http://www.wlxkc.cn/picture/1296374_13.png)
O-Arylation of 3-phenylpropanol (1a) and phenol (1ab) by triarylsulfonium triflates in the presence of CsOH.[a] [a] Reaction conditions: 1a or 1ab (0.2 mmol), 2 (0.3 mmol), CsOH (0.3 mmol), 1,4-dioxane (2 mL), 508C, N2, 24 h. Isolated yield. [b] Toluene (2 mL) as solvent and at 808C. [c] 808C. [d] 0.4 mmol scale (1ab).
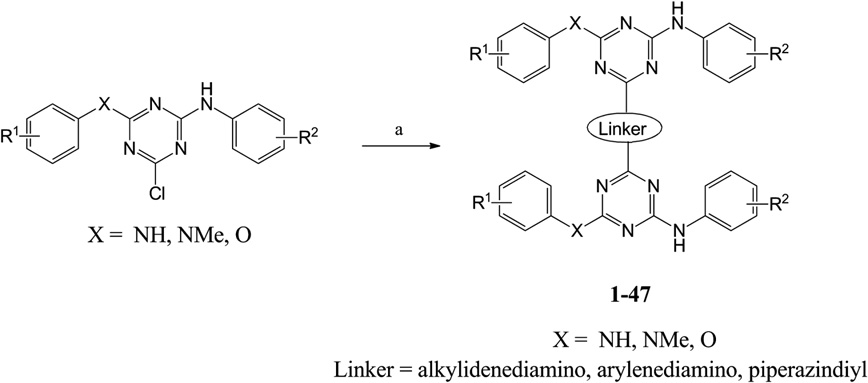
Synthesis of triazine dimers 1–47. Reagents and conditions: (a) diaminoalkanes/diaminobenzene/piperazine, DIPEA, dry dioxane, 110 °C.
Related Questions and Answers
A: The presence of methanol, a radical scavenger, significantly reduces the photocatalytic degradation of 1,4-dioxane by scavenging hydroxyl radicals (•OH), which are essential for the degradation process. In contrast, the addition of sodium chloride (NaCl) does not significantly affect the degradation rate, indicating that self-propulsion of the nanoparticles, which is inhibited by electrolytes, is not a primary mechanism for enhancing degradation in this system. A study concludes that the primary mechanism for enhanced degradation is the increased availability of hydroxyl radicals due to the Au coating and the specific wavelength of UV light used, rather than self-propulsion or the presence of electrolytes.
A: The presence of an Au coating on TiO₂ nanoparticles significantly enhances the photocatalytic degradation of 1,4-dioxane. The Au coating acts as an electron sink, sequestering photogenerated electrons from the conduction band of TiO₂ and preventing their recombination with holes. This leaves more holes available to react with water to form hydroxyl radicals, which are responsible for the degradation of 1,4-dioxane. Additionally, the Au coating increases the concentration of oxygen vacancies in TiO₂, which act as electron traps, further reducing electron-hole recombination and enhancing photocatalytic activity.
A: The wavelength of UV light plays a crucial role in the photocatalytic degradation of 1,4-dioxane using Au/TiO₂ Janus nanoparticles. The study found that 254 nm UV light resulted in significantly higher degradation rates compared to 365 nm UV light. This is because 254 nm light is more efficiently absorbed by TiO₂, creating more electron-hole pairs near the surface where they can react with water to form hydroxyl radicals. Additionally, the finite-element simulations showed that 254 nm light was absorbed preferentially near the TiO₂/water interface, whereas 365 nm light was absorbed deeper within the particle, requiring holes to travel a greater distance before reacting with water, thus reducing efficiency.
A: The Au/TiO₂ Janus nanoparticles significantly enhance the degradation of 1,4-dioxane under UV light by increasing the number of photogenerated holes in TiO₂ that can react with water to form hydroxyl radicals. This enhancement is due to two main factors: (1) the increased light absorption by TiO₂ under 254 nm UV light, which creates more electron-hole pairs near the surface, and (2) the sequestration of photogenerated electrons by the Au coating, preventing recombination and leaving more holes available to react with water. The optimal condition for degradation was achieved using 254 nm UV light at high intensity, resulting in a first-order rate constant of 0.138 min⁻¹, which is among the highest reported for 1,4-dioxane degradation without chemical additives.