4-(Dimethylamino)pyridine
CAS number: 1122-58-3
4-Dimethylaminopyridine is a dialkylarylamine and a tertiary amino compound. 4-Dimethylaminopyridine has been reported in Panax ginseng with data available.
Related images
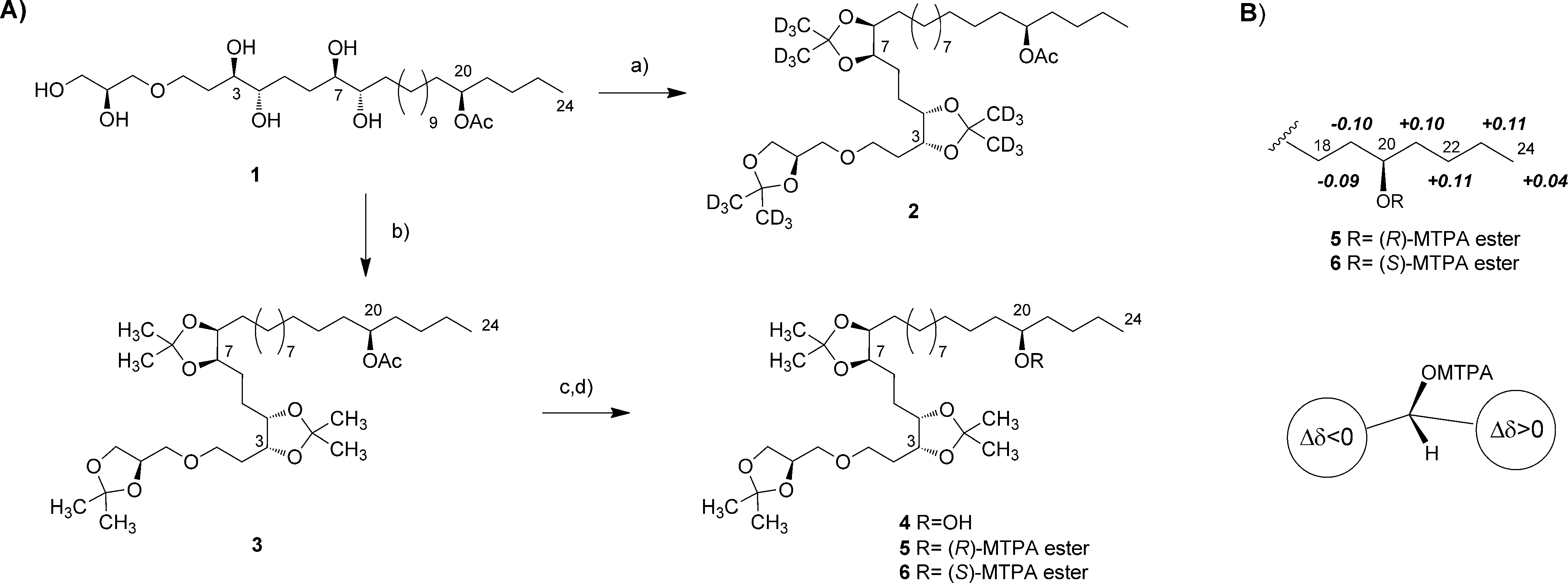
A) Synthesis of acetonide and MTPA derivatives of mycalol (1). B) Δδ (δS-δR) values (ppm) for selected protons of (R)-MTPA ester 5 and (S)-MTPA ester 6. Reagents and conditions: a) (CD3)2CO, I2, RT, 24 h; b) (CH3)2C(OCH3)2, PPTS, 758C, 6 h; c) Na2CO3, MeOH, 558C, 28 h; d) S-(-)- or R-(-)-MTPA chloride, DMAP, dry CH2Cl2, RT, overnight. Ac=acetyl, DMAP=4-dimethylaminopyridine, MTPA-Mosher’s acid; a- methoxy-a-(trifluoromethyl)phenylacetic acid, PPTS-pyridinium p-toluenesulfonate
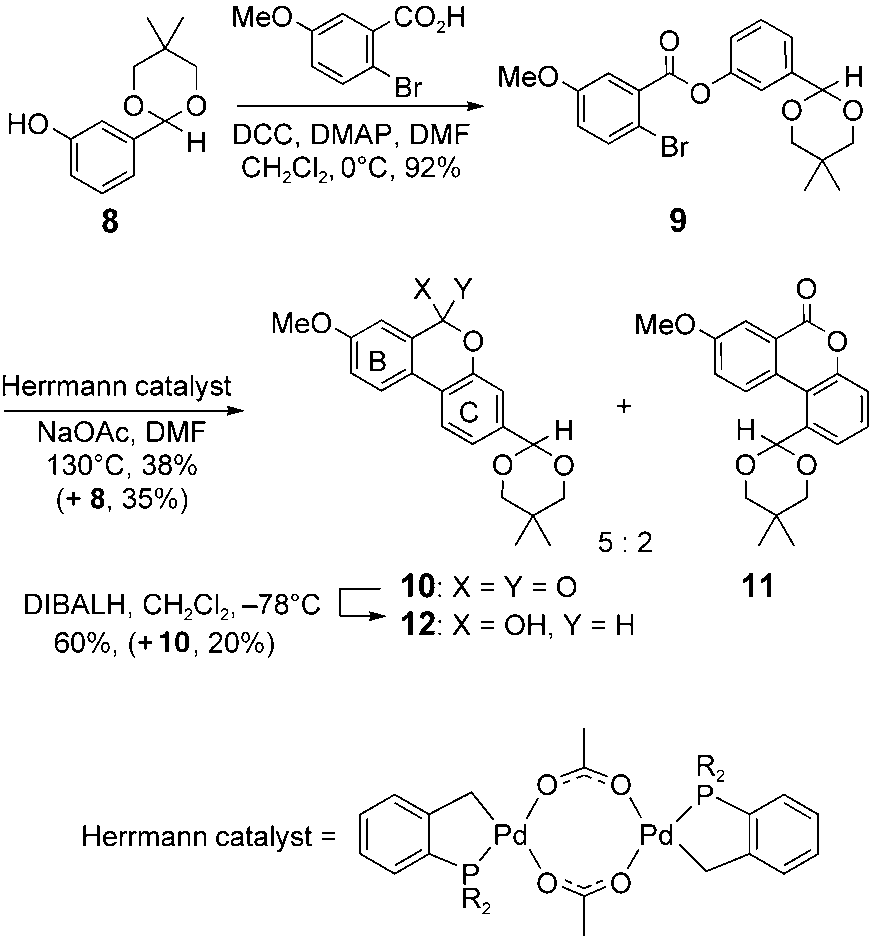
Synthesis of the BC ring system. DCC=dicyclohexylcarbo-diimide, DMAP=4-dimethylaminopyridine, DIBALH=diisobutylaluminum hydride, R=o-tolyl.

Preparation of cyclohexenol 16. DMAP=4-(dimethylamino)-pyridine, Cbz=benzyloxycarbonyl, THF=tetrahydrofuran, TBS=tert-butyldimethylsilyl, dppf=1,1'-bis(diphenylphosphino)ferrocene.
Related Questions and Answers
No related questions yet