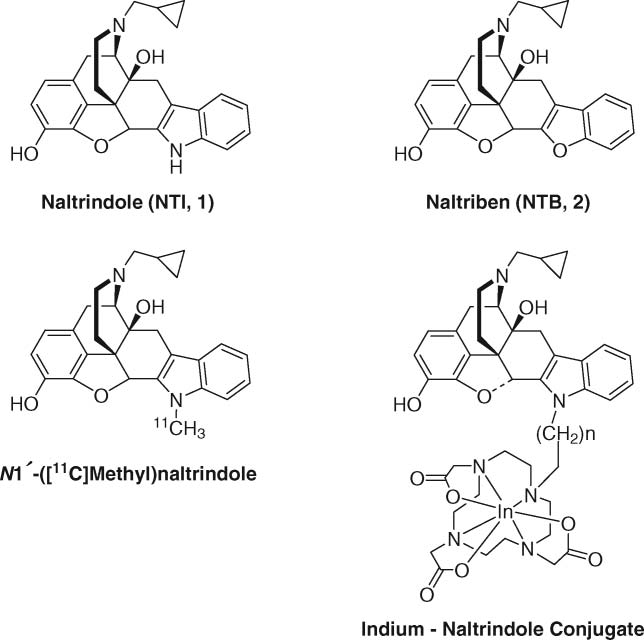
Naltrindole
CAS number: 111555-53-4
Naltrindole is a member of isoquinolines. Naltrindole (NTI), known as a delta opioid receptor antagonist, inhibited phorbol 12-myristate 13-acetate (PMA) induced superoxide (SO) release in polymorphonuclear leukocytes (PMNs).
Related images
Related Questions and Answers
A: The key findings from the binding affinity and functional activity assays showed that most of the synthesized NTI derivatives exhibited strong to moderate binding affinities for the DOR, with compound 9f (cyclopropylsulfonamide) being the most potent. Functional activity assays revealed that some compounds acted as full inverse agonists (e.g., 9f and 9g), while others were full agonists (e.g., 9e). These results highlight the diverse functional activities of NTI derivatives based on their N-substituents and suggest their potential use in studying the molecular mechanisms of DOR activation and inhibition.
A: The S=O moiety in sulfonamide-type NTI derivatives can function as a surrogate for the amide carbonyl moiety (C=O) in influencing functional activities. For instance, both amide-type (e.g., SYK-623) and sulfonamide-type (e.g., SYK-839) NTI derivatives with a cyclopropyl moiety exhibited inverse agonist activities. This suggests that the S=O group can compensate for the lack of a basic nitrogen atom, which is typically considered an essential pharmacophore in opioid ligands.
A: The binding affinities and functional activities of the NTI derivatives varied significantly based on their N-substituents. For example, compound 9f (cyclopropylsulfonamide) had the highest binding affinity for the DOR with a Ki value of 7.44 nM and exhibited strong inverse agonist activity. In contrast, compound 9e (phenethylsulfonamide) showed moderate binding affinity and full agonist activity. These results indicate that the N-substituent critically affects both the binding affinity and the functional activity of NTI derivatives for the DOR.
A: The N-substituent in NTI derivatives plays a crucial role in determining their functional activities for the DOR. For instance, cyclopropylsulfonamide 9f (SYK-839) was identified as a potent full inverse agonist, while phenethylsulfonamide 9e (SYK-901) exhibited full agonist activity with moderate potency. This suggests that the specific structure of the N-substituent can modulate the binding mode and conformational changes of the DOR, thereby influencing its functional activity.