Chlorobenzene
CAS number: 108-90-7
Chlorobenzene is a colorless, flammable liquid with an aromatic, almond-like odor. Some of it will dissolve in water, but it readily evaporates into air. It does not occur naturally in the environment. Chlorobenzene production in the United States has declined by more than 60% from its peak in 1960. It was used in the past to make other chemicals, such as phenol and DDT. Now chlorobenzene is used as a solvent for some pesticide formulations, to degrease automobile parts, and as a chemical intermediate to make several other chemicals.
Related images
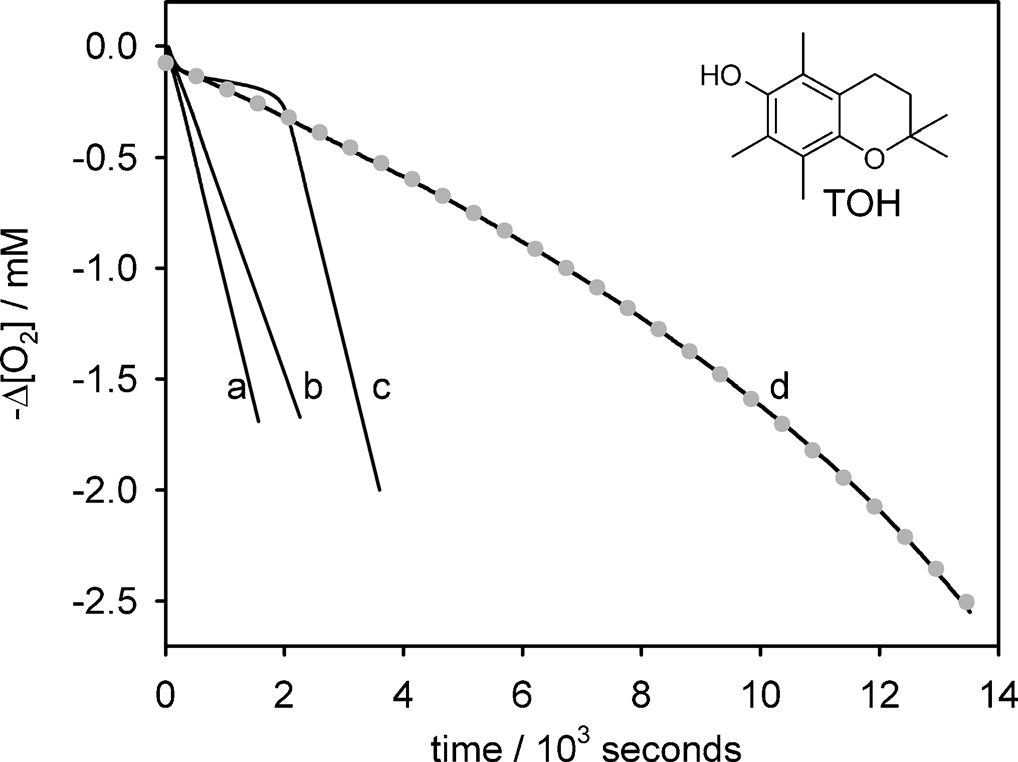
Oxygen consumption measured during autoxidation experiments in chlorobenzene initiated by AIBN (0.05m) at 308C with the following reactants: a) styrene (2.1m); b) CHD (cyclohexadiene, 0.26m); c) styrene (2.1m) and TOH (5 mm); d) CHD (0.26m) and TOH (6.2 mm).
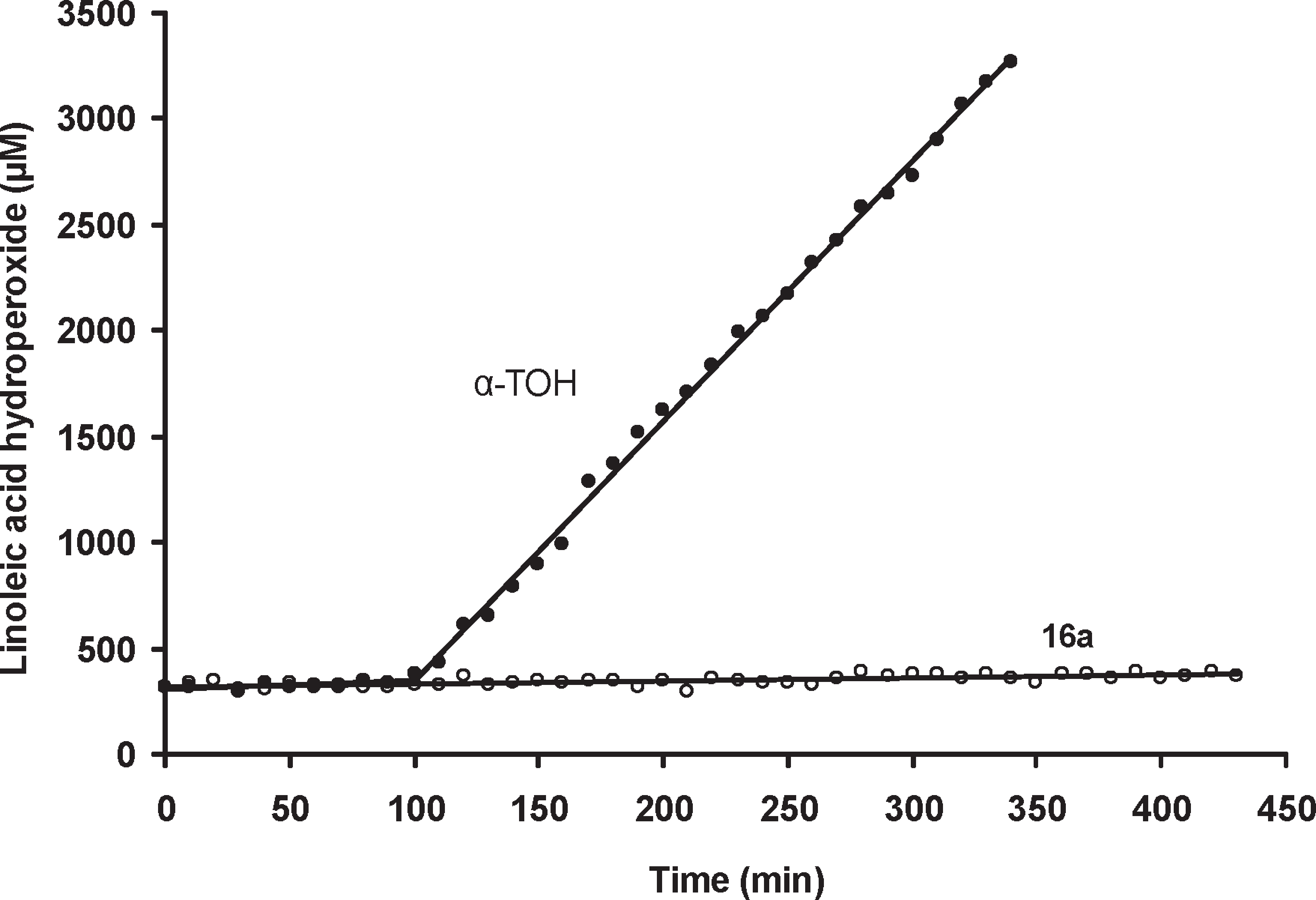
Peroxidation traces (linoleic acid hydroperoxide concentration vs time) recorded using compound 16a (40 μM) or R-TOH (40 μM) as antioxidants in the chlorobenzene layer in the presence of NAC (1 mM) in the aqueous phase.
Related Questions and Answers
No related questions yet