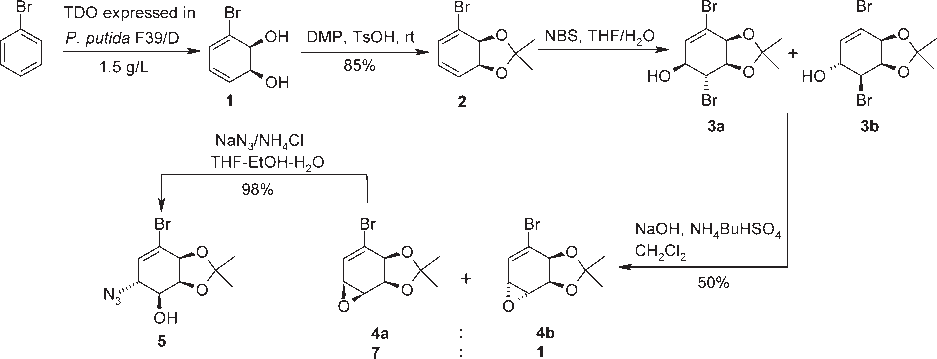
Bromobenzene
CAS number: 108-86-1
Bromobenzene is the simplest member of the class of bromobenzenes, that is benzene in which a single hydrogen has been substituted by a bromine. A liquid at room temperature (m.p. -30℃; b.p.760 156℃), it is used as a solvent, particularly for large-scale crystallisations, and for the introduction of phenyl groups in organic synthesis. It has a role as a non-polar solvent, a hepatotoxic agent and a mouse metabolite. It is a member of bromobenzenes, a bromoarene and a volatile organic compound.
Related images
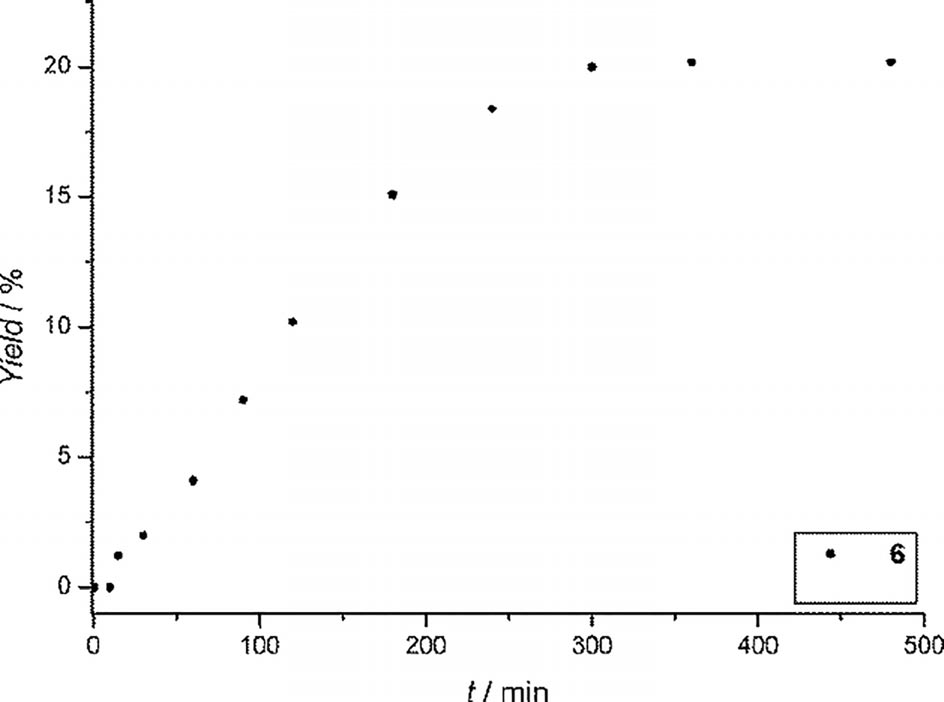
Reaction rate in the Mizoroki-Heck reaction of bromobenzene with n-butyl acrylate using 6 as precatalyst and adding mercury after 4 h.
Related Questions and Answers
No related questions yet